UDK: 616.853-085.216.6
Delova D.1,3, Mojsova Mijovska M.1,2, Burmuzoska M.2, Gavrilovska Brzanov A.2, Shuntov B.3, Kamilovski T.3
1Faculty of Medical Sciences “Goce Delchev” University, Shtip, Republic of North Macedonia
2 University Clinic for Traumatology, Orthopedic Diseases, Anesthesiology, Reanimation and Intensive Care Medicine and Emergency Department, Clinical Center “Mother Theresa”, Skopje, Republic of North Macedonia
3University Clinic of Neurosurgery, Clinical Center “Mother Theresa”, Skopje, Republic of North Macedonia
Abstract
Epilepsy is defined as a neurological disorder manifested by an excessive and abnormal electrical neuronal discharge. Refractory epilepsy still remains quite frequent condition, considered 30-40% of all patients with seizures, despite improvement in medication possibilities. Vagus Nerve Stimulator offers a neuromodulation approach when other treatment possibilities are exhausted. This minor procedure is safe but carries potential risks for anesthetic management and anesthesiologists should be aware of the physiological implications of the device and anticipate and manage possible complications.
Key Words: anesthesia management; epilepsy; vagus nerve stimulator.
Introduction
Epilepsy is defined as a neurological disorder manifested by an excessive and abnormal electrical neuronal discharge. It is affecting 50 million people worldwide with an incidence of 7.6 per 1,000 people (1). Refractory epilepsy still remains quite frequent condition, considered 30-40% of all patients with seizures, despite improvement in medication possibilities (1). More invasive modes of treatment include surgical resection of the epileptogenic foci or implantation of Vagus Nerve Stimulator (VNS), as the most common neuromodulation approach, when the former is not feasible (2). Careful selection of patients is mandatory, classifying patients eligible for surgical resection, deep brain stimulation or for VNS implantation (3). It is necessary to clearly explain to the patients the possible benefit of the procedure which according to many studies is seizure freedom in only 8% and 50% reduction of seizure frequency in 50-60% of the patients (1).
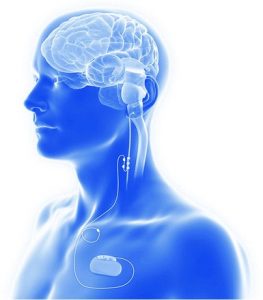
Figure 1. Schematic view of VNS components (American Journal of Neuroradiology May 2024, DOI: https://doi.org/10.3174/ajnr.A8235).
VNS System Components
Historically it was first investigated in 1938, but wasn’t implanted in humans until 1988, and then needed 9 more years to be FDA approved as an adjunctive treatment for epilepsy. Its implementation is accepted in adults and children older than 4 years, but off label also in younger than one year of age (1,4). Since then, many manufacturers’ modifications led to the development of the latest version of VNS. System components include a combination of stimulator (current pulse generator), single subcutaneous lead wire and platinum electrode which is wrapped around the vagus nerve with three helical coils (positive, negative and anchoring). This device can’t provide any sensing of peripheral muscular or central neuronal activity, so it can’t respond to current seizure activity and operates only by previously programmed parameters (5). These parameters are generally with electrical current of 1-2mA for 0.5ms and short time interval repetition of 20-30Hz for 30 seconds every 5 minutes (5,6).
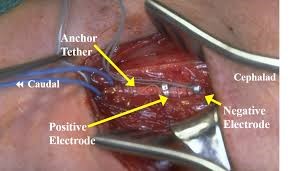
Figure 2. Intraoperative view of electrode positions and complete VNS set before implementation (https://www.cns.org/nexus/pediatric/case/vagal-nerve-stimulation-medically-intractable-epil).
VNS Surgical Implantation
After induction in anesthesia, the patient is placed in supine position with head slightly extended and turned right and elevated higher than the body. The left side of the neck and chest under the clavicle are prepared and draped. It is dissected throughout the neck layers, because the vagus nerve is nestled between the internal jugular vein and carotid artery in the carotid sheath. Left sided vagus nerve is preferred because the sinoatrial node is innervated by the right sided vagus nerve and placing it on the right side will contribute to arrhythmia as a side effect. Even on the left vagus nerve, it is important to place it inferior to the cardiac branches so the potential cardiac effects would be minimized. Aseptic measures must be followed to prevent infections, and also careful manipulations around the nerve and large vessels so catastrophic bleeding or nerve damage be avoided. Many complications are potentially described and are divided into two groups early or related to surgery and late or related to stimulation by the device. Early complications involve perioperative arrhythmia like bradycardia, complete atrio-ventricular block or even asystole, peritracheal hematoma, hoarseness because of nerve damage, dyspnea and left vocal cord paralysis (1). Late complications usually are due to infection or poor wound healing, delayed arrhythmia, neuralgia, obstructive sleep apnea, laryngopharyngeal dysfunction and battery malfunction (1,5,6).
Anesthetic Management during VNS Implantation
The procedure is mostly performed in general anesthesia but cases using a regional technique like combination of superficial and deep cervical plexus blocks and local anesthetic infiltration in the anterior chest wall are also described. Due to changes in pharmacokinetics of anesthetics, mainly by changes in their metabolism, their doses have to be adjusted. Antiepileptic medications are cytochrome p450 enzyme inductors which contribute to faster metabolism of opioids and need for higher doses. Also, there is an up-regulation on acetylcholine receptors in the neuromuscular junction requiring also a higher dose of neuromuscular blockers to achieve satisfactory block. Due to poor control of seizures and very high risk of perioperative seizure, there is a recommendation for proceeding with the antiepileptic medications in the morning before surgery. All potential triggers that can provoke seizures like hypocarbia should be managed and avoided (5). Anesthetics like propofol and thiopental are safe to use, but drugs like ketamine are still questionable (5). There is a risk of massive bleeding due to close interconnection of the nerve with internal jugular vein and carotid artery, so blood products should be available if needed. All patients should be closely monitored because of the risk of early postoperative complications like seizures, peritracheal hematoma or vocal cord paralysis but also hemodynamic changes due to testing of the stimulator.
Mechanism of Action
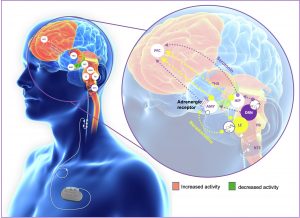
Large number of studies are conducted but the exact mechanism of VNS is still debatable. Research data shows a highly complex vagal afferent network that is proposed as a modulation place for the stimulator (7). The left vagus nerve is proposed as a more favorable site for placement because it has a smaller impact on heart function, giving fibers that innervate the AV node, in comparison to the right vagus nerve that has effect on SA node in the heart. The nerve itself is quite complex and contains afferent and efferent fibers, partly myelinated A and B and partly unmyelinated C-fibers (5,7). Most of the afferent fibers terminate in Nucleus Tractus Solitarius (NTS) as a railway station for the information carried by the largest cranial nerve in the body. Then NTS, with its wide range of projections, transmits impulses to several key structures in the brainstem like the noradrenergic locus coeruleus (LC), serotoninergic raphe nucleus (RN), cerebellum, periaqueductal gray matter and parabrachial nuclei (PBN) (5,7). All these structures project to many other higher centers in the brain like the amygdala, the hypothalamus, the thalamus and limbic system (7). The data confirms that with stimulation of 1mA, alteration of neurotransmitters occurs like extracellular noradrenaline increase and also dopamine, serotonin, γ-amino butyric acid (GABA) and glycine (7).
Figure 3. Afferent Vagal network and proposed structures (https://www.neuromodulationjournal.org/article/S1094-7159(22)01222-3/fulltext).
Our Center Experience
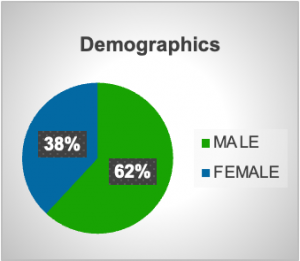
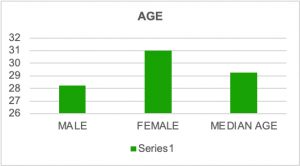
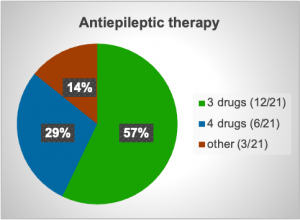
In the period from 2021 till 2025, twenty-one VNS were implanted in patients with refractory epilepsy with different etiologies and all with similar treatment with multiple antiepileptic medications in high doses. Many reasons were noted as contributors to epilepsy like trauma, tuberous sclerosis-related epilepsy, tumor resection, febrile convulsions in early childhood and Sy Dravet. Demographically 13 patients were male with median age 28 years and 8 were female with median age 31 years. More than a half of them had a need for treatment with three antiepileptics, in total 12 out of 21 patient or 57% and 6 out of 21 or 28.57% were treated with four different antiepileptics as a combined therapy. After surgery a reduction or even cessation of seizures were noted in many of the patients after a few months, but not a reduction of drug dose. Some of the isolated complications that were noted were transitory hoarseness, numbness in the left side of the face, redness of the left eye and mild hypertension noted after VNS implantation.
Graph 1. Demographic characteristics of patients.
Graph 2. Antiepileptic therapy (Patients using 3 different medications, 4 different medications and other).
Conclusion
Vagus nerve stimulation (VNS) continues to be an effective adjunctive treatment for patients who have drug-resistant epilepsy, particularly in situations where resective surgery is not an option. Despite the fact that the procedure is minimally invasive, it requires careful perioperative planning due to the potential risks associated with the anesthetic management. The experience of our center reinforces its safety profile and feasibility, with many patients experiencing a reduction in seizure burden. However, the outcomes vary, and long-term success is dependent on appropriate patient selection, clear preoperative communication, and awareness of device-related implications in future surgical or anesthetic settings. Furthermore, as the role of VNS expands, particularly into non-epilepsy indications, it is essential to take a structured and multidisciplinary approach in order to maximize its effectiveness.
References:
- Gouveia FV, Warsi NM, Suresh H, Matin R, Ibrahim GM. Neurostimulation treatments for epilepsy: Deep brain stimulation, responsive neurostimulation and vagus nerve stimulation. Neurotherapeutics. 2024 Apr;21(3):e00308. doi: 10.1016/j.neurot.2023.e00308. Epub 2024 Jan 4. PMID: 38177025; PMCID: PMC11103217.
- González HFJ, Yengo-Kahn A, Englot DJ. Vagus Nerve Stimulation for the Treatment of Epilepsy. Neurosurg Clin N Am. 2019 Apr;30(2):219-230. doi: 10.1016/j.nec.2018.12.005. PMID: 30898273; PMCID: PMC6432928.
- Broderick L, Tuohy G, Solymos O,et al. Management of vagus nerve stimulation therapy in the peri-operative period: Guidelines from the Association of Anaesthetists: Guidelines from the Association of Anaesthetists. Anaesthesia. 2023 Jun;78(6):747-757. doi: 10.1111/anae.16012. Epub 2023 Apr 25. PMID: 37096456.
- Orosz I, McCormick D, Zamponi N, et al. Vagus nerve stimulation for drug-resistant epilepsy: a European long-term study up to 24 months in 347 children. Epilepsia. 2014 Oct;55(10):1576-84. doi: 10.1111/epi.12762. Epub 2014 Sep 17. PMID: 25231724.
- Hatton KW, McLarney JT, Pittman T, Fahy BG. Vagal nerve stimulation: overview and implications for anesthesiologists. Anesth Analg. 2006 Nov;103(5):1241-9.doi: 10.1213/01.ane.0000244532.71743.c6. PMID: 17056962.
- Giordano F, Zicca A, Barba C, Guerrini R, Genitori L. Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia. 2017 Apr;58 Suppl 1:85-90. doi: 10.1111/epi.13678. PMID: 28386925.
- Carron R., Roncon P., Lagarde S., Dibué M., Zanello M., Bartolomei F. 2023. Latest Views on the Mechanisms of Action of Surgically Implanted Cervical Vagal Nerve Stimulation in Epilepsy.Neuromodulation 2023; 26: 498–506.
- Shetty A, Pardeshi S, Shah VM, Kulkarni A. Anesthesia considerations in epilepsy surgery. Int J Surg. 2016 Dec;36(Pt B):454-459. doi: 10.1016/j.ijsu.2015.07.006. Epub 2015 Jul 15. PMID: 26188082.