UDK: 616.62-006.6-056.7:577.218
TELOMERASE GENE EXPRESSION IN URINARY BLADDER CARCINOMA
Ambardjieva M.1, Stankov V.2, Panov S.4, Gavrilovska Brzanov A.3, Janchulev J.2, Saidi S.2
1 University Hospital of Surgical Disease, “St. Naum Ohridski”, “Ss Cyril and Methodius” University, Faculty of Medicine, Skopje, Republic of North Macedonia
2 University Clinic of Urology, Medical Faculty, “Ss Cyril and Methodius” University, Skopje, Republic of North Macedonia.
3 Faculty of Natural Sciences and Mathematics, Institute of Biology, “Ss Cyril and Methodius” University,Skopje, Republic of North Macedonia
4 University Clinic for Traumatology, Orthopedics, Anesthesiology, Reanimation, Intensive Care and Emergency Department – Skopje, Department of Anesthesiology, Reanimation and Intensive Care, “Ss Cyril and Methodius” University, Skopje.
Abstract
Urinary bladder carcinoma (UBC) is the most prevalent malignancy of the urinary tract and is associated with high recurrence and mortality rates. Histologically, urothelial carcinoma is the predominant form, accounting for nearly 90% of all bladder cancer cases. One of the most frequent molecular alterations in malignant neoplasms, including UBC, is the abnormal activation of the telomerase gene.
This study aimed to quantitatively assess the expression of the TERT gene using Real-Time PCR in tissue samples obtained from patients with UBC. In total 34 patients, with histopathological confirmed urothelial bladder carcinoma, were included, along with a control group of 17 individuals without bladder malignancy.
The mean TERT gene expression level in the low-grade subgroup was 1.268 ± 0.472, compared to 2.137 ± 0.942 in the high-grade subgroup. This difference was statistically highly significant (p< 0.0001). Logistic regression analysis revealed that patients with high-grade UBC were 7.39 times more likely to exhibit elevated TERT gene expression compared to those with low-grade UBC (p< 0.001). The area under the ROC curve (AUC) was calculated to be 7.89, indicating that the model is highly effective in predicting tumor grade based on TERT expression levels.
The study established a positive correlation between histological grade and TERT gene expression levels. These findings suggest that molecular detection of abnormal telomerase transcriptional activity could serve as a useful auxiliary biomarker in the personalized management of patients with bladder carcinoma.
Key Words: gene expression, urinary bladder cancer, telomerase, TERT.
Introduction
Urinary bladder carcinoma (UBC) is the most common malignancy of the urinary system and is characterized by high recurrence and mortality rates. Histologically, urothelial carcinoma is the most prevalent type, accounting for approximately 90% of all bladder cancer cases.
Urinary bladder carcinoma accounts for approximately 3% of newly diagnosed cancers globally, with men affected up to four times more than women (1, 2), The highest incidence is reported in Southeastern Europe (26.6/100,000 men), and rates are expected to rise due to aging populations and increased tobacco use (3).
According to the Cancer Registry of the Institute of Public Health of North Macedonia (4), there were 239 newly diagnosed cases of UBC, yielding an incidence rate of 11.52 per 100,000 inhabitants. In 2020, 137 deaths were attributed to bladder cancer (101 men and 36 women).
A major challenge in managing bladder tumors is their tendency to recur and progress to higher stages and grades. Furthermore, treatment response is highly variable, as UBCs display diverse phenotypes with differing responses to surgery, chemotherapy, radiotherapy, immunotherapy and other treatments. Modern urological science within the medical field seeks to bridge the gap between tumor phenotype and genotype to better guide clinical decision-making.
To that end, there is a trend toward centralizing and standardizing preoperative diagnostic procedures for all patients with UBC. After initial transurethral resection (TUR), a treatment strategy is developed based on both the phenotypic and genotypic characteristics of the tumor.
There is a strong interest, both scientifically and clinically, in identifying molecular and genetic markers that correlate with clinical and histopathological features of UBC. These biomarkers offer potential for use in differential diagnosis, disease prognosis, therapy selection and patient monitoring.
Telomeres are repetitive DNA-protein structures at the ends of eukaryotic chromosomes that protect them from degradation, fusion and genomic instability (5, 6). Due to the end-replication problem, telomeres shorten with each cell division, eventually leading to cellular senescence or apoptosis in normal somatic cells (7). Telomerase, a specialized enzyme composed of an RNA template (coded by hTR or hTERC gene) and a catalytic subunit telomerase reverse transcriptase (coded by gene TERT), counteracts telomere shortening by adding telomeric repeats to chromosome ends (8, 9). While telomerase is active in germline, stem and some immune cells, it is typically inactive in most of the somatic cells.
In cancer, telomerase is abnormally reactivated, allowing cells to bypass senescence and achieve unlimited replicative potential- a hallmark of malignant transformation (10, 11). The TERT gene plays a central role in this process, and its expression is regulated by transcriptional and epigenetic mechanisms, including promoter binding sites and GC-rich regions that influence chromatin remodeling and methylation (12). Telomerase activation occurs in over 80% of human cancers through various telomere maintenance mechanisms, such as TERT gene mutations, rearrangements, amplifications, alternative splicing and transcription factor binding alterations, which are often tumor-type and tissue-specific (13, 14).
Our study aims to quantitatively assess TERT gene expression in patients with urothelial bladder carcinoma and examine its correlation with key clinical and pathological parameters. The ultimate goal is to evaluate the potential of abnormal telomerase activity as a supportive molecular marker in guiding personalized treatment strategies for bladder cancer patients.
Materials and Methods
This study involved the quantitative determination of TERT gene expression using Real-Time PCR in tissue samples obtained from patients diagnosed with urothelial bladder carcinoma (UBC). The investigation included 34 patients with histopathological confirmed UBC and a control group of 17 patients without malignant bladder disease.
Inclusion criteria in the study required primary tumors with a histopathological confirmed diagnosis of urothelial bladder carcinoma and written informed consent from the patient. Exclusion criteria included absence of confirmed urothelial carcinoma, presence of a different tumor type, incomplete or missing key clinical data, insufficient quality of isolated DNA/RNA, age under 18 years or refusal to provide informed consent.
For each patient, selected demographic data (age and gender) and histological grade of tumor differentiation were collected. The study was approved by the Research Ethics Committee at the Doctoral School of the Faculty of Medicine in Skopje.
Tissue samples were obtained either during transurethral resection (TUR). For control purposes, samples of healthy bladder mucosa were collected from patients undergoing surgery for non-malignant pathologies with no history of bladder malignancy.
Each sample (200–300mg in weight) was preserved and transported in RNA stabilization solution (RNA Later) and stored at −80°C until analysis. Total RNA was isolated from tumor tissues using an automated nucleic acid extractor and a dedicated RNA extraction kit (Genolution).
RNA concentrations were measured using a Qubit 3.0 Fluorometer.
Quantitative TERT gene expression levels were assessed using a two-steps process involving reverse transcription and real-time PCR (qRT-PCR) amplification with fluorescent TaqMan probes, performed on the OneStep Real-Time PCR System (Applied Biosystems).
Total RNA was extracted from frozen TUR-obtained tissue samples using TRI-reagent. Complementary DNA (cDNA) synthesis was performed via reverse transcription using High-Capacity cDNA Reverse Transcription Kits (Thermo-Fisher), following the manufacturer’s instructions.
The following oligonucleotide primers and TaqMan probes were used:
- TERT gene:
- Forward primer (A): 5′-GCA TTG GAA TCA GAC AGC AC-3′,
- Reverse primer (R): 5′-CCA CGA CGT AGT CCA TGT TC-3′,
- TaqMan probe: 5′FAM-CGC CCT GCT GAC GTC CAG AC-NFQ-3′.
- Reference gene GAPDH:
- Forward primer (A): 5′-ATG GGT GTG AAC CAT GAG AA-3′,
- Reverse primer (R): 5′-GTG CTA AGC AGT TGG TGG TG-3′,
- TaqMan probe: 5′FAM-CCT CAA GAT CAT CAG CAA TGC CTC C-NFQ-3′.
The amplification protocol included:
- Polymerase activation: 10 minutes,
- 40 cycles of:
- Denaturation at 95°C for 15 seconds,
- Combined annealing and elongation at 70°C for 1 minute.
A negative control (ddH₂O) was used for each master mix to validate the assay.
TERT gene expression levels were calculated using the Livak method (2^−ΔCt), relative to the expression of the housekeeping gene GAPDH. The ΔCt value was determined by subtracting the Ct (threshold cycle) value of GAPDH from the Ct of TERT for each sample.
The differential expression of TERT was presented as the relative fold-change compared to GAPDH, expressed as log₁₀(RQ) for consistency across samples.
Statistical methods were applied to determine correlations between clinical/ histopathological data and molecular genetic findings. Descriptive statistics were used to analyze and present relevant demographic and clinical characteristics of the patients.
Correlation between the clinical and histopathological parameters and the molecular genetic results, was assessed using logistic regression analysis. Parametric values with normal distribution were evaluated using a two-tailed Student’s t-test, whereas deviations from normal distribution were analyzed using the non-parametric Mann-Whitney U-test.
The suitability of TERT gene expression levels for logistic regression analysis was verified using the Hosmer–Lemeshow goodness-of-fit test. The odds ratio (OR) and 95% confidence interval (CI) were calculated, with a significance threshold of p< 0.05.
All statistical analyses were performed using XLSTAT 2016 and Microsoft Excel 2016 software.
This study presents data from 34 patients with histologically confirmed urothelial bladder carcinoma (UBC), along with 17 control samples from individuals without malignant bladder disease.
Tumor Grade Distribution
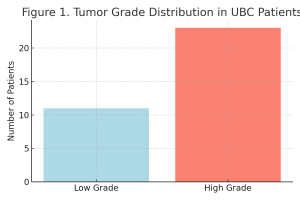
Based on histological evaluation, 11 patients were classified as having low-grade tumors and 23 as having high-grade tumors (Table 1, Figure 1).
Table 1. Histological Grade Distribution among UBC Patients.
| Grade | n | % |
| Low Grade | 11 | 32.35% |
| High Grade | 23 | 67.65% |
| Total | 34 | 100.00% |
Figure 1. Distribution of Histological Grades in UBC Patients.
Gender Distribution
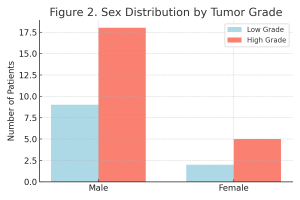
Gender distribution in both histological subgroups is shown in Table 2 and Figure 2.
Table 2. Gender Distribution by Tumor Grade.
| Gender | Low Grade (n/%) | High Grade (n/%) | Fisher’s Exact Test (p) |
| Male | 9 (81.82%) | 18 (78.26%) | 1.000 |
| Female | 2 (18.18%) | 5 (21.74%) | |
| Total | 11 (100.00%) | 23 (100.00%) |
Figure 2. Gender Distribution Across Tumor Grades.
Age Distribution
The age distribution of the two subgroups is shown in Table 3.
Table 3. Age Structure by Tumor Grade.
| Parameter (years) | Low Grade | High Grade | Student’s t-test (p) |
| n | 11 | 23 | 0.745 |
| Mean Age | 66.18 | 63.04 | |
| SD | 10.57 | 8.14 | |
| Min Age | 47 | 46 | |
| Max Age | 80 | 76 |
There was no statistically significant difference in age or gender distribution between the two tumor grade groups (p> 0.05), supporting their comparability for further analysis.
TERT Gene Expression Levels
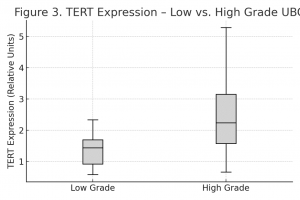
TERT gene expression levels, normalized to the GAPDH reference gene, were evaluated across the two histological subgroups. The results are presented in Table 4 and Figure 3.
Table 4. TERT Gene Expression by Tumor Grade.
| hTERT Expression | Low Grade | High Grade | Mann-Whitney Test (p) |
| n | 11 | 23 | < 0.001 |
| Mean | 1.268 | 2.137 | |
| SD | 0.472 | 0.942 | |
| Min | 0.422 | 0.671 | |
| Max | 2.156 | 4.996 |
Figure 3. TERT Expression in Low vs. High-Grade UBC.
The data clearly demonstrate significantly higher TERT gene expression levels in high-grade tumors compared to low-grade ones. The mean expression level in the high-grade subgroup was 2.137 ± 0.942, while in the low-grade subgroup, it was 1.268 ± 0.472. This difference is statistically highly significant (p< 0.001), confirming a strong and proportional correlation between TERT gene expression and histological grade in urothelial bladder carcinoma.
Logistic Regression Analysis
Logistic regression analysis confirmed that increased TERT expression is significantly associated with higher tumor grades. Patients with high-grade tumors were 7.39 times more likely to exhibit elevated TERT expression (p= 0.001).
Table 5. Logistic Regression – TERT Expression and Tumor Grade.
| Parameter | β Coefficient | SE | Wald χ² | p-value | OR (95% CI) |
| Grade | 1.073 | 0.318 | 11.36 | 0.001 | 7.39 (2.07 – 25.85) |
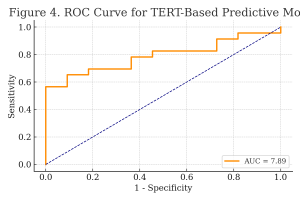
Figure 4. ROC Curve of the Predictive Model.
The area under the ROC curve (AUC) of 7.89 indicates excellent model accuracy in distinguishing high-grade from low-grade tumors based on TERT expression.
The model’s predictive performance, based on TERT expression levels, is summarized in Table 6. It correctly classified tumor grades in 76.47% of cases.
Table 6. Predictive Accuracy of TERT-Based Model.
| Metric | Value |
| Sensitivity | 91.30% |
| Specificity | 63.64% |
| Overall Accuracy | 76.47% |
Discussion
Telomerase activity is abnormally elevated in over 80% of malignant tumors and is considered to be a key factor in cellular immortalization and tumor progression (15). Numerous studies have confirmed the reactivation of telomerase in various cancers, including urothelial bladder carcinoma (UBC), where its activity may contribute to the malignant transformation of urothelial cells (16).
In this study, the transcriptional activity of telomerase was assessed through quantitative measurement of TERT gene expression levels in tumor tissue from 34 patients with UBC. The results were normalized against GAPDH and compared to 17 control samples from healthy bladder mucosa. A statistically significant increase in TERT expression was observed in tumor tissues compared to controls, which aligns with previously published studies on bladder cancer (17, 18, 19, 20, 21).
Notably, TERT expression levels were significantly higher in high-grade tumors compared to low-grade ones (mean values: 2.137 vs. 1.268, p< 0.001), suggesting a correlation between telomerase activity and tumor aggressiveness. These findings are consistent with earlier reports that linked elevated telomerase expression with higher histological grade and advanced pathological stage (22, 23).
Furthermore, logistic regression analysis showed that patients with high-grade tumors were 7.39 times more likely to exhibit increased TERT expression than those with low-grade tumors, with excellent predictive value supported by an AUC of 7.89. The model demonstrated a sensitivity of 91.30% and correctly classified tumor grade in over 76% of the cases.
Previous studies also suggest that TERT overexpression is associated with poor prognosis in various tumor types, including colorectal cancer (24), supporting its broader relevance as a prognostic marker.
Collectively, the current findings reinforce the potential clinical value of TERT gene expression in stratifying bladder cancer patients and guiding treatment strategies based on tumor biology.
Conclusion
This study confirmed a positive correlation between the histological grade of urothelial bladder carcinoma and the quantitative expression levels of the TERT gene. The patients with high-grade tumors exhibited significantly higher TERT expression than those with low-grade tumors.
The results suggest that molecular detection of abnormal telomerase transcriptional activity may serve as a supportive molecular biomarker in the personalized management of bladder cancer. Its use could aid in risk stratification, prognosis, and therapeutic decision-making, particularly in settings where tumor aggressiveness needs to be evaluated beyond standard histopathology.
References:
- IARC, Cancer Today. Estimated number of new cases in 2020, worldwide, both sexes, all ages. 2021. [Access date: March 2022] https://gco.iarc.fr/today/online-analysis-table.
- Sung H, Ferlay J, Siegel RL, Laversanne M et al. Global Cancer Statistics 2020,
- GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338.
- Регистар за рак во Република Северна Македонија. Институт за јавно здравје, Скопје, 2021.
- Blackburn EH. The end of the (DNA) line. Nat Struct Biol. 2000 Oct;7(10):847-50. doi: 10.1038/79594.
- De Lange T. How telomeres solve the end-protection problem. Science. 2009 Nov 13;326(5955):948-52. doi: 10.1126/science.1170633.
- Huffman KE, Levene SD, Tesmer VM et al.Telomere shortening is proportional to the size of the G-rich telomeric 3′-overhang. J Biol Chem. 2000 Jun 30;275(26):19719-22. doi: 10.1074/jbc.M002843200.
- Greider CW. Molecular biology. Wnt regulates TERT–putting the horse before the cart. Science. 2012 Jun 22;336(6088):1519-20. doi: 10.1126/science.1223785.
- Dwyer J, Li H, Xu D, Liu JP. Transcriptional regulation of telomerase activity: roles of the the Ets transcription factor family. Ann N Y Acad Sci. 2007 Oct;1114:36-47. doi: 10.1196/annals.1396.022.
- Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT). Gene. 2012 May 1;498(2):135-46. doi: 10.1016/j.gene.2012.01.095. Epub 2012 Feb 13.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646-74. doi: 10.1016/j.cell.2011.02.013.
- Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008 Aug;99(8):1528-38. doi: 10.1111/j.1349-7006.2008.00878.x.
- Gaspar TB, Sá A, Lopes JM et al. Telomere Maintenance Mechanisms in Cancer. Genes (Basel). 2018 May 3;9(5):241. doi: 10.3390/genes9050241.
- Dratwa M, Wysoczańska B, Łacina et al. TERT-Regulation and Roles in Cancer Formation. Front Immunol. 2020 Nov 19;11:589929. doi: 10.3389/fimmu.2020.589929.
- Shay JW, Wright WE. Telomeres and telomerase: three decades of progress. Nat Rev Genet. 2019 May;20(5):299-309. doi: 10.1038/s41576-019-0099-1.
- Tsatsakis A, Oikonomopoulou T, Nikolouzakis TK et al. Role of telomere length in human carcinogenesis (Review). Int J Oncol. 2023 Jul;63(1):78. doi: 10.3892/ijo.2023.5526. Epub 2023 May 26.
- Okumura A, Mizuno I, Nagakawa O et al. Telomerase activity is correlated with lower grade and lower stage bladder carcinomas. Int J Urol. 2004 Dec;11(12):1082-6. doi: 10.1111/j.1442-2042.2004.00960.x.
- Jafri MA, Ansari SA, Alqahtani MH et al. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016 Jun 20;8(1):69. doi: 10.1186/s13073-016-0324-x.
- Bravaccini S, Sanchini MA, Granato AM et al. Urine telomerase activity for the detection of bladder cancer in females. J Urol. 2007 Jul;178(1):57-61. doi: 10.1016/j.juro.2007.03.025. Epub 2007 May 11.
- Zhang B, Bai YX, Ma HH et al. Silencing PinX1 compromises telomere length maintenance as well as tumorigenicity in telomerase-positive human cancer cells. Cancer Res. 2009 Jan 1;69(1):75-83. doi: 10.1158/0008-5472.CAN-08-1393.
- Morii A, Komiya A, Okumura A et al. Telomerase activity in bladder cancer tissue. Exp Ther Med. 2010 Jan;1(1):85-88. doi: 10.3892/etm_00000015. Epub 2010 Jan 1.
- Lin Y, Miyamoto H, Fujinami K et al. Telomerase activity in human bladder cancer. Clin Cancer Res. 1996 Jun;2(6):929-32.
- Takihana Y, Tsuchida T, Fukasawa M et al. Real-time quantitative analysis for human telomerase reverse transcriptase mRNA and human telomerase RNA component mRNA expressions as markers for clinicopathologic parameters in urinary bladder cancer. Int J Urol. 2006 Apr;13(4):401-8. doi: 10.1111/j.1442-2042.2006.01300.x .
- Tatsumoto N, Hiyama E, Murakami Y et al. High telomerase activity is an independent prognostic indicator of poor outcome in colorectal cancer. Clin Cancer Res. 2000 Jul;6(7):2696-701.