UDK: 615.243.6.06:616.65-089.87-089.168
Petrova Chemerski N.1, Gavrilovska-Brzanov A.1, Panovska Petrusheva A.1, Temelkovska Stevanov M.¹, Jovanovski Srceva M.¹, Shabani B.²
1 University Clinic for Traumatology, Orthopedic Disease, Anesthesiology, Reanimation and Intensive Care Medicine and Emergency Department, Faculty of Medicine, “Ss Cyril and Methodius” University, Skopje, RN Macedonia
2 University Clinic for Urology, Faculty of Medicine, “Ss Cyril and Methodius” University, Skopje, RN Macedonia
Abstract
Introduction
Postoperative nausea and vomitus (PONV) are serious complication subsequent to laparoscopic radical prostatectomy. Although the prevention of PONV states that a single bolus dose of one antiemetic drug is recommended, combining treatment with two or more drugs or continuous antiemetic drug infusion is more effective.
Objectives
To evaluate and compare the occurrence and severity of postoperative nausea and vomiting (PONV) in the postoperative phases in patients undergoing laparoscopic prostatectomy who receive various antiemetic prophylaxes.
Materials and Methods
This prospective, comparative study included 40 patients who underwent laparoscopic radical prostatectomy, equally divided into two groups: one receiving intraoperative antiemetic prophylaxis with a combination of two agents Group FM (intra operative receiving metoclopramide and famotidine), and the other with a single agent (receiving intra- and postoperative continuous infusion of Dexmedetomidine) Group DEX. The dry retching and nausea were assessed at five postoperative time points: immediately after extubating, and at 2, 4, 12 and 24 hours.
Results
Postoperatively, PONV was identified in total 8 (20%) patients in the two groups. The incidence of PONV in group DEX was lower, and it was 15% or 3 patients with grade 1 PONV, versus 25% or 5 patients with grade 2 PONV in group FM.
Conclusion
Our results indicate that Dexmedetomidine could significantly lower the occurrence and gradus of PONV compared, with combination of Famotidine and Metoclopramide after laparoscopic prostatectomy.
Key Words: dexmedetomidine; metoclopramide; postoperative nausea and vomitus.
Introduction
Over the years, surgical techniques have evolved significantly, with minimally invasive approaches like laparoscopic prostatectomy (LPR) becoming increasingly common. Compared to open surgery, LPR offers several advantages: reduced trauma, less postoperative pain and feasibility as a day-case procedure (1).
However, the creation of pneumoperitoneum using carbon dioxide can cause notable hemodynamic, renal and respiratory effects due to elevated intra-abdominal pressure (2). The occurrence of postoperative nausea and vomiting (PONV) as one of the most common postoperative complications of LPR has incidence of 40–70% (3). This is largely triggered by CO₂ insufflation, which activates serotonin receptors in the gut and stimulates the chemoreceptor trigger zone (4). Risk factors for PONV include non-smoking patients, general anesthesia, laparoscopic interventions, history of motion sickness and stress-prone personality (5).
Despite modern antiemetic strategies, PONV remains one of the most frequent complications in the first 24 hours after laparoscopic surgery (6,7). It can lead to prolonged recovery, delayed discharge, increased healthcare costs, and complications such as appetite loss, dehydration, electrolyte imbalance, wound dehiscence, esophageal rupture and even pneumothorax (8,9). The incidence rises to 53–70% in high-risk patients (Apfel score ≥3) (9). Therefore, crucial for improved patients’ outcomes and reducing hospital stay costs, is early prevention and treatment of PONV.
Monitoring PONV is especially important in high-risk groups, influenced by factors like gender, surgery type and duration, anesthesia duration, CO₂ exposure, and even emotional stress in the recovery room (10). The vomiting reflex is mediated through the vomiting center and the chemoreceptor trigger zone (CTZ) in the medulla oblongata (11). Other risk factors include age >50, female gender, infections, uremia, migraines, hypercalcemia and anxiety (9,10,11). Specific surgeries such as abdominal laparoscopy, gynecologic procedures, strabismus, and ear surgery are also associated with higher PONV rates (10,11).
Prophylaxis depends on risk level. Single-drug therapy is recommended for moderate-risk patients (Apfel score 1–2), while high-risk patients benefit more from combination therapies involving multiple antiemetic drug classes (10). The most common used drugs are: butyrophenones, serotonin antagonists, steroids, H₂-receptor antagonists, anticholinergics and phenothiazines. Famotidine, metoclopramide and dexmedetomidine are among the drugs used. Although famotidine has no direct antiemetic effect, it inhibits histamine H₂ receptors in gastric parietal cells, reducing acid secretion. Given intravenously, it reaches peak effect in 30 minutes and lasts 10–12 hours (12). Metoclopramide, a dopamine receptor antagonist with prokinetic effects, acts both centrally and peripherally. Its onset is within 15 minutes and duration 1–2 hours (13). Dexmedetomidine, an α₂-agonist, exerts antiemetic effects by reducing sympathetic tone and perioperative opioid use. It begins acting within 5–10 minutes, peaks at 15–30 minutes, and lasts 60–120 minutes. With a half-life of 2 hours, its pharmacokinetic profile supports use in both intraoperative and early postoperative settings (14).
Hence, the main objective of this small study was to compare the prophylactic effects of famotidine and metoclopramide combination alongside dexmedetomidine in reducing PONV in patients undergoing laparoscopic prostatectomy.
Materials and Methods
Study Design: This study was designed as a prospective comparative clinical evaluation conducted at the University Clinic of Urology, Skopje, and the University Clinic for Anesthesiology, Reanimation and Intensive Care Medicine Faculty of Medicine, “Ss Cyril and Methodius” University, Skopje, RN Macedonia. The evaluation included a total of 40 male patients scheduled for laparoscopic prostatectomy (LPR) between January and December 2024. The enrollment of the patients scheduled for LPR in the study was conducted after obtaining informed consent. The study protocol received ethical approval from the Institutional Review Board (IRB) and ethical committee from the medical faculty, and written informed consent was obtained from all participants before inclusion in the investigation.
Patients’ Selection Criteria: Inclusion criteria incorporated male patients aged 50–75 years with histologically confirmed prostate carcinoma requiring LPR, ASA Class I and II and 180-minutes maximum duration of surgery. Patients with pre-existing psychiatric illnesses, Parkinson’s disease, motion sickness, or a history of chemotherapy were excluded to minimize confounding factors affecting PONV. Excluding factors were identified allergies to the medications in this study but also patients with EF ≤30%, coronary occlusions ≥ 50%, bradycardia ≤ 50 min, MAP ≤ 65mmHg and atrioventricular block grade Ⅱ, due to the use of Dexmedetomidine.
The patients were randomly divided into two groups (combination of famotidine and metoclopramide – group FM and dexmedetomidine – group DEX) using a cubull randomization. All patients received complete monitoring, including noninvasive blood pressure (NIBP), heart rate (PR), oxygen saturation (SpO2) and body temperature measurements.
Group FM (Famotidine + Metoclopramide): Patients in this cohort were given 20mg of famotidine and 10mg of metoclopramide intraoperatively, immediately following intubation.
Group DEX (Dexmedetomidine): Patients in this cohort were administered dexmedetomidine at a dosage of 0.4 micrograms per kilogram per hour (μg/kg/h) during the intraoperative phase, thereafter followed by a decreased dosage of 0.1μg/kg/h for 8 hours postoperatively.
Examined Parameters: PONV was assessed through serial of physical examinations and questionnaires in five different time points:
- T1 – Immediately after the extubating,
- T2 – 2 h after the surgery,
- T3 – 4 h after the surgery,
- T4 – 12 h after the surgery,
- T5 – 24 h after the surgery.
After the completion of surgery, the first physical exam and questionnaire for T1 was taken in the operating room, after which the patients entered the recovery room where the following checkups and surveys, including questions about the scour of nausea and vomiting and hemodynamic parameters of the patient in T2, T3 and T4 were completed. The last exam and questionary for T5 were taken in the patient’s room. All patients with vomiting scores of 2 and > 5 were treated with ondansetron (4mg I.V. 1cc). Ondansetron is one of the imperative drugs in preventing PONV due to surgery and chemotherapy. This serotonin receptor antagonist exhibits its anti-vomiting effects by inhibiting 5-hydroxytryptamine type 3 (5-HT3) receptors in the vomiting center and the compressor starting area (15). Finally, the obtained data were analyzed by statistical software SPSS 23 and the data were presented in the form of statistical tables and charts.
Assessment of PONV: Evaluation of the risk for PONV was made with use of the Apfel risk score and determining the grade and severity of PONV was made using the most recent grade and impact scale respectively.
Table 1. Apfel’s risk score and the PONV impact scale.
| Apfel’s risk factors | |
| Non-smoker | 1 |
| Postoperative opioids | 1 |
| History of PONV | 1 |
| Female gender | 1 |
| Total score | 0-4 (Total score ≥ 3 clinically significant) |
| PONV impact scale calculator | |
| Dry-retching episodes | |
| Not at all | 0 |
| Once | 1 |
| Twice | 2 |
| Three or more times | 3 |
| Nausea episodes | |
| Not at all | 0 |
| Sometimes | 1 |
| Often or most of the time | 2 |
| All the time | 3 |
| Total score (≥5 clinically significant) | 0-6 |
One of the tools that have proven to be effective in assessing the patient’s baseline risk PONV and also has implications in the protocol for patient-specific antiemetic prophylaxis is the Apfel’s risk score. The factors included in the Apfel’s score are postoperative use of opioids, non-smoker status, female gender and previous history of PONV or motion sickness. Correspondingly, all these risk factors contribute to elevating the incidence of PONV by about 20% (9). Each risk factor is given a score of 1, the total score being 4. PONV is classified as grades 0, 1 and 2. Grades 1 and 2 are considered as PONV (16).
PONV impact scale calculator is a tool that assesses the clinical significance of the PONV, and it is based on the patient’s assessment of the impact of their nausea on their postoperative recovery and the number of experienced vomiting. It includes questions about the presence of nausea and its quantity and questions about presence and the number of vomiting. A score ≥5 is considered clinically significant (16).
Table 2. PONV grade.
| PONV grade | Patient’s response |
| 0 | Without PONV |
| 1 | Nausea without vomitus |
| 2 | Nausea with vomiting (≤ 3 times/day) |
| 3 | Vomiting ≥ 3 times /day |
PONV grade is determined by a four-point (0-3) scoring system, with PONV score 0= no signs of nausea and retching; 1= episodes of sickness and retching; 2= vomiting one or two times in a period of 30 min; 3= vomiting more than two times in a period of 30 min (9,16).
Anesthesia Protocol: Per the protocol, all patients received conventional preoperative preparation, which included a minimum fasting period of six hours and the maintenance of normothermia. Two hours before surgery, patients received 5mg of oral diazepam as premedication. Upon entering the operating room, patients were subjected to continuous hemodynamic monitoring utilizing the Datex-Ohmeda S/5 Avance (Helsinki, Finland), which recorded the following parameters: electrocardiography (ECG), heart rate (HR), non-invasive blood pressure (NIBP) and invasive mean arterial pressure (MAP) at five-minute intervals, along with oxygen saturation (SpO₂), capnography (end-tidal CO₂ – EtCO₂), fraction of inspired oxygen (FiO₂), and intra-abdominal pressure (9–12 mmHg) through the laparoscopic insufflation system. An intravenous cannula was inserted in each patient, and a crystalloid infusion was delivered at a rate of 6–12ml/kg/hr during anesthesia. Before induction, patients received preoxygenation with 100% oxygen at a flow rate of 6 L/min for three minutes. General endotracheal anesthesia was initiated with 0.04mg/kg midazolam, 0.002mg/kg fentanyl, 1–2mg/kg propofol, and 0.6mg/kg rocuronium. After loss of consciousness and the stoppage of spontaneous respiration, patients were manually ventilated, and endotracheal intubation was conducted two minutes post-administration of rocuronium. Mechanical ventilation commenced utilizing the Datex-Ohmeda S/5 Avance in Pressure-Controlled Ventilation – Volume Guarantee (PCV-VG) mode, with a tidal volume of 6–8ml/kg, a gas mixture comprising 50% oxygen and 50% air, an inspiratory-to-expiratory ratio (I:E) of 1:2, a respiratory rate calibrated to sustain EtCO₂ between 35–45mmHg, and a positive end-expiratory pressure (PEEP) of 5cm H₂O. Anesthesia was sustained by a balanced method utilizing remifentanil (0.05–1µg/kg/min) and sevoflurane at 1 MAC, ensuring mean arterial pressure remained within ±20% of baseline values. A nasogastric tube was inserted for decompression, intraoperative normothermia was sustained using forced-air warming blankets, and anti-embolism pumps were utilized for all patients to avert thromboembolic problems. Postoperative treatment encompassed standardized analgesia and fluid resuscitation according to institutional procedure (2024 NICE guidelines). 25-30ml/kg/day of water and 1mmol/kg/day of sodium, potassium, and chloride, in accordance to the British Consensus Guidelines on IV Fluid for Adult Surgical Patients. GIFTASUP advised a low volume maintenance fluid of 1-1.5ml/kg/hr, with fluid boluses of 0.5ml/kg/hr for the resuscitation of postoperative oliguria, along with serial evaluations of postoperative nausea and vomiting (PONV) using the PONV grade and impact scale for up to 24 hours postoperatively. Patients suffering from PONV were treated in accordance to the most recent recommendations and guidelines, employing a multimodal approach with antiemetic medications, and utilizing ondansetron as the “gold standard” for PONV management (17).
All patients with vomiting scores of 2 and > 5 were treated with ondansetron 4mg i.v. /1cc.
Statistical Analysis: The data were examined utilizing SPSS software (version 27.0, IBM Corp.). Continuous variables were assessed for normalcy with the Shapiro-Wilk test. Parametric data were represented as mean ± standard deviation (SD) and evaluated via the paired t-test, whereas non-parametric data were provided as a median with interquartile range (IQR) and compared using the Mann-Whitney U test. A p-value of less than 0.05 was deemed statistically significant.
Results
Out of a total of 40 patients who underwent laparoscopic prostatectomy, 3 patients (15%) from the DEX group had Apfel score of 3 with a 60% possibility of developing PONV, and from the FM group, one patient (5%) had Apfel score of 3, which means a 60% possibility of PONV, and one patient from the same group had an Apfel score 4 (5%) with an 80% possibility of PONV. From those patients with significant predictive Apfel score from the FM group (from the PONV impact scale calculator), 5 patients had PONV in T1, 4 patients in T2, 2 patients in T3, 1 patient in T4, and no patients in T5. Furthermore, out of these patients, 1 had grade 0 PONV, 2 had grade 1 PONV and 2 had grade 2 PONV. The number of patients with significant predictive Apfel score from the DEX group (score ≥5 from the PONV impact scale calculator) was 3 patients in T1, 1 patient in T2, and no patients in T3, T4 and T5. Out of these patients, 2 had grade PONV 1.
Table 3 presents the demographic characteristics of the study population: a mid-age value of 61 (50-72) years in the FM group and 65 (55-75) in the DEX group. Average BMI of 30 (21-39) kg/m² in the FM group and 31 (29-32) kg/m² in the DEX group. The average duration of LRP in the FM group was 155 (135-175) minutes and 150 (138-172) minutes in the DEX group. There were 6 patients, or 30%, who were non-smokers in the FM group and a total of 3 non-smoker patients in the DEX group. According to ASA score there were 14 patients (70%) with an ASA Ⅰ score in the FM group and the same number of 14 (70%) ASA Ⅰ patients in the DEX group. The number of ASA Ⅱ patients in both groups was also the same, 6 (30%). ASA Ⅲ and ASA Ⅳ patients were excluded from the study.
Table 3. Patients’ demographic characteristic (N=40).
| Parameter | Famotidine + Metoclopramide Group FM n(%) | Dexmedetomidine
Group DEX n(%) |
| Age (years), median (IQR) | 61 (50-72) | 65 (55-75) |
| BMI (kg/m2), median (IQR) | 30 (21-39) | 31 (29-32) |
| Average duration of surgery(min), median (IQR) | 155 (135-175) | 150 (138-172) |
| Nonsmoker | 6 (30) | 3(15) |
| ASA score | ||
| Ⅰ | 14(70) | 14(70) |
| Ⅱ | 6(30) | 6(30) |
| Ⅲ | 0(100) | 0(100) |
| Ⅳ | 0(100) | 0(100) |
| Previous PONV | 4 (20) | 3(15) |
| Previous motion sickness | 3(15) | 2(10) |
| Apfel score | ||
| 1 | 4(20) | 0(100) |
| 2 | 3(15) | 0(100) |
| 3 | 1(5) | 3(15) |
| 4 | 1(5) | 0(100) |
There were 4 (20%) patients with previous PONV in the FM group and 3 (15%) in the DEX group. Previous motion sickness was noted in 3 patients (15%) in the FM group and in 2 patients (10%) in the DEX group.
Apfel score for predicting the PONV was 1 for 4 patients (20%) in the FM group and none in the DEX group. Apfel score 2 for 3 (15%) of patients in the first and none in the latter group. Apfel score 3 was noted in 1 patient (5%) in the FM, versus 3 (15%) patients in the DEX group. And finally, Apfel score 4 was noted just in 1 (5%) patient in the first FM group.
The difference between the two groups regarding mean age, BMI and mean duration of surgery was noteworthy (P ≥ 0.05).
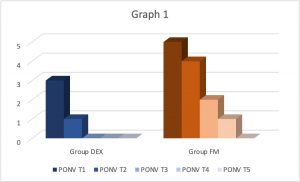
Graph 1. Comparing vomiting and nausea scores in T1, T2, T3, T4, T5 of DEX group versus FM group.
Giving the results in Graph 1 there was a substantial difference in the frequency and the grade of nausea and vomiting among the two groups, and the incidence of PONV exhibited a significant decrease in the DEX group as compared to FM groups (p˂0.005).
Table 4. Comparison of the incidence and severity of PONV between the two groups.
| Group DEX | Group FM |
| PONV T1 = 3 | PONV T1 = 5 |
| PONV T2 = 1 | PONV T2 = 4 |
| PONV T3 = 0 | PONV T3 = 2 |
| PONV T4 = 0 | PONV T4 = 1 |
| PONV T5 = 0 | PONV T5 = 0 |
| Grade 0 = 0 | Grade 0 = 1 |
| Grade 1 = 2 | Grade 1 = 2 |
| Grade 3 = 0 | Grade 2 = 2 |
The incidence of PONV in the DEX group was lower and it was 15 % with grade 1 versus 25 % with grade 2 in the FM group.
Discussion
Anesthesiologists play a crucial role in determining an appropriate pharmacological regimen for managing PONV. One of the symptoms of PONV that could occur during the first 24 hours after general anesthesia, nausea, is defined as a feeling of unpleasant agitation and discomfort in the abdomen, followed by inevitable occurrence of vomiting (18). Despite the certain advancements in the field of new drugs for PONV, nausea and vomiting are still persistent as a common complaint after general anesthesia, with frequency of occurrence from 20% to 30% of the patients who undergo general anesthesia within 24 hours of surgery (18, 19). The incidence of nausea and vomiting post-surgery depends upon numerous circumstances, including the surgical procedure (laparoscopy, strabismus correction, ear surgery, gynecological surgery), the anesthetic drugs, and also the anesthetic employed in the procedure (17,18,19).
Laparoscopic prostatectomies are now increasingly being performed. Shorter hospital stay is the advantage of this procedure, but PONV may lengthen stay in hospital and increase the treatment cost. The results of previous studies were in accordance to our study.
The research by Masilamani involving 100 patients revealed an average hospital stay of 1.19 days, much lower than the 3-4 days typically required for open radical prostatectomy (20). Previous research by Parra-Sanchez et al., demonstrated that patients experiencing PONV had a significantly prolonged stay in the post-anesthesia care unit compared to those without PONV. This supports the notion that PONV not only affects patient’s comfort but also has a measurable impact on recovery efficiency and resource utilization. Furthermore, there was meaningfully a notable difference in the nursing time required for patients with PONV than the patients without PONV with statistically significant numbers. Subsequently, the total cost of postoperative recovery for PONV patients was greater and therefore was associated with an adjusted incremental total cost. The postoperative quality of life in PONV patients was worse (49% of patients with PONV rated quality high in four domains vs 94% of patients without PONV (21). These results align with those obtained in our investigation.
Prevention and treatment of PONV alongside providing suitable scales and drugs has been one of the important concerns of anesthesiologists over the years. The drugs that are used as prophylaxis or for the treatment of PONV include serotonin antagonists, anticholinergics, butyrophenones, phenothiazines, steroids, and histamine H2-receptor antagonists. Although the recommendations stand for a single-drug prophylactic administration, combining treatment with two or more drugs from different classes or continuous infusion of anti-emetic drugs is more effective than single medicine for high-risk patients (4,10). The results from using some of these anti-emetic drugs: famotidine, metoclopramide and dexmedetomidine —in our study were in agreement with previous studies, like the study of Nesek-Adam V. that included 160 patients in which none of the patients form the dexamethasone plus metoclopramide group patients (p< 0.05 versus groups 1 and 2) and only one of the dexamethasone group patient (p< 0.05 versus group 1) required antiemetic rescue, vice the four patients in the metoclopramide group and six patients in the placebo group that and PONV (1). Another study also contributed to the results that the combination of two antiemetic drugs was found to meaningfully decrease the incidence of PONV compared to single antiemetic drug. Furthermore, in a series of 140 patients the results were as follows: significantly lower rate of PONV in patients receiving a combination of metoclopramide and droperidol than those administered metoclopramide alone or placebo. Those receiving two-dose droperidol alone also had a statistically significantly lower incidence of PONV compared to metoclopramide and placebo (4).
A score to rate clinically important PONV from a patient’s point of view was developed and validated by Wengritzsky et coauthors and named the PONV intensity scale. The practicality of the PONV intensity scale led to the development and validation of a simplified score by Myles and Wengritzky, named the PONV impact scale (22). It consists of two questions directed to patient (Table 1). A score of ≥5 from the two questions defines clinically significant PONV. It has been reported that patients perceive PONV to be more distressing than pain, which necessitates assessment of its incidence to ensure that it is not undertreated, and that effective measures are undertaken to address it (9). Weilbach with coauthors, published a prospective study with 93 patients from 2006 that highlighted that in the group with an Apfel score of 3, PONV occurred in 59.7% of the patients and in the Apfel score group of 4, in 91.3% of all patients. The incidence of PONV corresponded to the predicted values of 60% for Apfel 3 and 80% for Apfel 4. The conclusion was that the Apfel score is a useful and simple tool for stratification of patients with high risk for PONV (9, 10, 23).
In terms of which agents have more efficacy and low cost for PONV prophylaxis, more research is required. Our study evidences that there is a significant difference and decrease in the frequency and the grade of nausea and vomiting among the use of dexmedetomidine compared to the combined use of famotidine and metoclopramide (p˂0.005). It was also confirmed in the meta-analysis with 6,480 patients by the author Liang X. The results confirmed that dexmedetomidine reduces postoperative nausea (Risk Ratio (RR) = 0.61, 95% confidence interval (CI): 0.50 to 0.73) and vomiting compared to placebo, with an effective dose of 0.5μg/kg (RR = 0.46, 95% CI: 0.34 to 0.62) and 1.0μg/kg (RR = 0.29, 95% CI: 0.12 to 0.75), respectively. Moreover, its application lowered intraoperative requirement of fentanyl. The results of this meta-analysis showed the superior dexmedetomidine efficacy to placebo, all related to a reduced intraoperative opioid consumption (14).
Likewase, in an updated meta-analysis trial from 2023 with total 18 trials involving 2018 patients and 15 updated of previous studies, Zhao W et al. supported our findings with the results of PONV incidence in DEX group that is lower than that in the control group (OR=0.49, 95% CI: 0.36 to 0.67), and significantly decreased perioperative opioid consumption in the DEX group (standard mean difference (SMD)=-1.04, 95% CI: -1.53 to -0.54). Moreover, the length of hospitalization (SMD=-2.29, 95% CI: -4.31 to -0.28) and the extubating time (SMD=-0.75, 95% CI: -1.26 to -0.25) in DEX group were shorter. In final conclusion, Dexmedetomidine could decrease the occurrence of PONV in adult patients under general anesthesia and promote the recovery after surgery (24).
Furthermore, in another meta-analysis from 2017, Jin S et al. give more evidence that support our results that dexmedetomidine could decrease the occurrence of PONV after general anesthesia. PONV in the dexmedetomidine group was meaningfully lower versus the placebo group (0.56, 95% CI: 0.46, 0.69). Perioperative fentanyl consumption in the dexmedetomidine group was also reduced significantly (P < 0.00001). Subgroup analysis showed that dexmedetomidine administration by loading dose plus continuous infusion, by loading dose, or just by continuous infusion, the incidence of PONV during general anesthesia was decreased significantly, and therefore dexmedetomidine administered in continuous infusion mode has the advantage to prevent PONV as well as reducing side effects such as bradycardia and hypotension (25).
This aligns with our findings of significant decrease of the incidence of PONV in the DEX group compared to the FM group (p˂0.005). The incidence of PONV in the DEX group was lower, and it was 15% with grade 1 versus 25% with grade 2 in the FM group.
Despite the valuable insights gained from this study, one main limitation should be acknowledged: the relatively small sample size that may limit the generalizability of our findings, and larger multicenter studies are needed to confirm these results.
Conclusion
Our findings indicate that the investigated antiemetic drugs (dexmedetomidine, famotidine and metoclopramide) are effective in reducing postoperative nausea and vomiting in patients undergoing laparoscopic prostatectomy. It should be noted, on the other hand, that the antiemetic effect of dexmedetomidine was substantially more powerful when compared to the combination of famotidine and metoclopramide.
References:
- Nesek-Adam V, Grizelj-Stojcić E, Rasić Z, Cala Z, Mrsić V, Smiljanić A. Comparison of dexamethasone, metoclopramide, and their combination in the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Surg Endosc. 2007 Apr;21(4):607-12. doi: 10.1007/s00464-006-9122-7. Epub 2007 Feb 7. PMID: 17285386.
- Sodha S, Nazarian S, Adshead JM, Vasdev N, Mohan-S G. Effect of Pneumoperitoneum on Renal Function and Physiology in Patients Undergoing Robotic Renal Surgery. Curr Urol. 2016 Feb;9(1):1-4. doi: 10.1159/000442842. Epub 2016 Feb 10. PMID: 26989363; PMCID: PMC4789951.
- Szarvas S, Chellapuri RS, Harmon DC, Owens J, Murphy D, Shorten GD. A comparison of dexamethasone, ondansetron, and dexamethasone plus ondansetron as prophylactic antiemetic and antipruritic therapy in patients receiving intrathecal morphine for major orthopedic surgery. Anesth Analg. 2003 Jul;97(1):259-63, table of contents. doi: 10.1213/01.ane.0000066310.49139.2a. PMID: 12818978.
- Nesek-Adam V, Grizelj-Stojcić E, Mrsić V, Smiljanić A, Rasić Z, Cala Z. Prophylactic antiemetics for laparoscopic cholecystectomy: droperidol, metoclopramide, and droperidol plus metoclopramide. J Laparoendosc Adv Surg Tech A. 2004 Aug;14(4):212-8. doi: 10.1089/lap.2004.14.212. PMID: 15345158.
- Fujii Y. The utility of antiemetics in the prevention and treatment of postoperative nausea and vomiting in patients scheduled for laparoscopic cholecystectomy. Curr Pharm Des. 2005;11(24):3173-83. doi: 10.2174/1381612054864911. PMID: 16178752.
- Wu SJ, Xiong XZ, Cheng TY, Lin YX, Cheng NS. Efficacy of ondansetron vs. metoclopramide in prophylaxis of postoperative nausea and vomiting after laparoscopic cholecystectomy: a systematic review and meta-analysis. Hepatogastroenterology. 2012 Oct;59(119):2064-74. doi: 10.5754/hge11811. PMID: 22282127.
- Kamali, A., Ahmadi, L., Shokrpour, M.,Pazuki, S. Investigation of ondansetron, haloperidol, and dexmedetomidine efficacy for prevention of postoperative nausea and vomiting in patients with abdominal hysterectomy. Open Access Macedonian Journal of Medical Sciences, 2018; 6(9): 1659–1663. https://doi.org/10.3889/oamjms.2018.366 .
- Eidi M, Kolahdouzan K, Hosseinzadeh H, Tabaqi R. A comparison of preoperative ondansetron and dexamethasone in the prevention of post-tympanoplasty nausea and vomiting. Iran J Med Sci. 2012 Sep;37(3):166-72. PMID: 23115448; PMCID: PMC3470085.
- Weilbach C, Rahe-meyer N, Raymondos K, Weissig A, Scheinichen D, Piepenbrock S. Postoperative nausea and vomiting (PONV) : usefulness of the Apfel-score for identification of high risk patients for PONV. Acta Anaesthesiol Belg. 2006;57(4):361-3. PMID: 17236637.
- Darvall J, Handscombe M, Maat B, So K, Suganthirakumar A, Leslie K. Interpretation of the four risk factors for postoperative nausea and vomiting in the Apfel simplified risk score: an analysis of published studies. Can J Anaesth. 2021 Jul;68(7):1057-1063. English. doi: 10.1007/s12630-021-01974-8. Epub 2021 Mar 15. PMID: 33721198.
- Jabalameli, Mitra et al. “Treatment of postoperative nausea and vomiting after spinal anesthesia for cesarean delivery: A randomized, double-blinded comparison of midazolam, ondansetron, and a combination.” Advanced Biomedical Research 1 (2012): n. pag.
- Nozaki C, Morioka N, Kinoshita M, Takada M, Ozaki M. [Can preoperative administration of H2 blocker reduce the incidence of postoperative nausea and vomiting (PONV)?]. Masui. 2007 Oct;56(10):1161-4. Japanese. PMID: 17966618.
- De Oliveira GS Jr, Castro-Alves LJ, Chang R, Yaghmour E, McCarthy RJ. Systemic metoclopramide to prevent postoperative nausea and vomiting: a meta-analysis without Fujii’s studies. Br J Anaesth. 2012 Nov;109(5):688-97. doi: 10.1093/bja/aes325. Epub 2012 Sep 25. PMID: 23015617.
- Liang X, Zhou M, Feng JJ, Wu L, Fang SP, Ge XY, Sun HJ, Ren PC, Lv X. Efficacy of dexmedetomidine on postoperative nausea and vomiting: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2015 Jun 15;8(6):8450-71. PMID: 26309498; PMCID: PMC4538099.
- Zhang D, Shen Z, You J, Zhu X, Tang QF. Effect of ondansetron in preventing postoperative nausea and vomiting under different conditions of general anesthesia: a preliminary, randomized, controlled study. Ups J Med Sci. 2013 May;118(2):87-90. doi: 10.3109/03009734.2013.768315. Epub 2013 Feb 26. PMID: 23441598; PMCID: PMC3633335.
- Myles PS, Wengritzky R. Simplified postoperative nausea and vomiting impact scale for audit and post-discharge review. Br J Anaesth. 2012 Mar;108(3):423-9. doi: 10.1093/bja/aer505. Epub 2012 Jan 29. PMID: 22290456.
- Yoo JH, Jeon IS, Chung JW, Ryoo JH, You GW, Kim SI. Comparison of palonosetron and ondansetron to prevent postoperative nausea and vomiting in women using intravenous patient-controlled analgesia. Anesth Pain Med (Seoul). 2020 Jan 31;15(1):28-34. doi: 10.17085/apm.2020.15.1.28. PMID: 33329786; PMCID: PMC7713856.
- Rüsch D, Eberhart LH, Wallenborn J, Kranke P. Nausea and vomiting after surgery under general anesthesia: an evidence-based review concerning risk assessment, prevention, and treatment. Dtsch Arztebl Int. 2010 Oct;107(42):733-41. doi: 10.3238/arztebl.2010.0733. Epub 2010 Oct 22. PMID: 21079721; PMCID: PMC2977990.
- Ghosh S, Rai KK, Shivakumar HR, Upasi AP, Naik VG, Bharat A. Incidence and risk factors for postoperative nausea and vomiting in orthognathic surgery: a 10-year retrospective study. J Korean Assoc Oral Maxillofac Surg. 2020 Apr 30;46(2):116-124. doi: 10.5125/jkaoms.2020.46.2.116. PMID: 32364351; PMCID: PMC7222617.
- Masilamani MKS, Sukumar A, Cooke PW, Rangaswamy C. Role of multimodal anaesthetic in post-operative analgesic requirement for robotic assisted radical prostatectomy. Urologia. 2022 Feb;89(1):90-93. doi: 10.1177/03915603211031869. Epub 2021 Aug 2. PMID: 34338049.
- Parra-Sanchez I, Abdallah R, You J, Fu AZ, Grady M, Cummings K 3rd, Apfel C, Sessler DI. A time-motion economic analysis of postoperative nausea and vomiting in ambulatory surgery. Can J Anaesth. 2012 Apr;59(4):366-75. doi: 10.1007/s12630-011-9660-x. Epub 2012 Jan 6. PMID: 22223185.
- Wengritzky R, Mettho T, Myles PS, Burke J, Kakos A. Development and validation of a postoperative nausea and vomiting intensity scale. Br J Anaesth. 2010 Feb;104(2):158-66. doi: 10.1093/bja/aep370. Epub 2009 Dec 26. PMID: 20037151.
- Pierre S, Benais H, Pouymayou J. Apfel’s simplified score may favourably predict the risk of postoperative nausea and vomiting. Can J Anaesth. 2002 Mar;49(3):237-42. doi: 10.1007/BF03020521. PMID: 11861340.
- Zhao W, Li J, Wang N, Wang Z, Zhang M, Zhang H, Liu M, He J, Yu D. Effect of dexmedetomidine on postoperative nausea and vomiting in patients under general anaesthesia: an updated meta-analysis of randomised controlled trials. BMJ Open. 2023 Aug 1;13(8):e067102. doi: 10.1136/bmjopen-2022-067102. PMID: 37527891; PMCID: PMC10394554.
- Jin S, Liang DD, Chen C, Zhang M, Wang J. Dexmedetomidine prevent postoperative nausea and vomiting on patients during general anesthesia: A PRISMA-compliant meta analysis of randomized controlled trials. Medicine (Baltimore). 2017 Jan;96(1):e5770. doi: 10.1097/MD.0000000000005770. PMID: 28072722; PMCID: PMC5228682.