UDK: 616.379-008.64:618.3]:616.153.915
Todorova B.1, Tosheska –Trajkovska K.2, Bitoska I.1, Ahmeti I.1, Milenkovik T.1
1University Clinic for Endocrinology, Diabetes and Metabolic Diseases, Clinical Center “Mother Theresa” Faculty of Medicine, “Ss Cyril and Methodius” University, Skopje, Republic of North Macedonia
2Institute of Medical and Experimental Biochemistry, Faculty of Medicine, “Ss Cyril and Methodius” University, Skopje, Republic of North Macedonia
Abstract
Introduction:Gestational diabetes mellitus, characterized by glucose intolerance during pregnancy, poses heightened risks to both the mother and fetus. It emerges due to increased insulin resistance during pregnancy. Adipose tissue plays a vital role in regulating various biological processes through the secretion of adipokines, which influence both pregnancy and the development of gestational diabetes. Hyperlipidemia, a well-known contributor to atherosclerosis, directly impacts the onset of cardiovascular diseases. Pregnancy leads to an increase in serum levels of total cholesterol and triglycerides, driven by increased levels of hormones such as estrogens, progesterone and lactogen. Material and Methods: This study presents prospective clinical research involving 65 individuals, excluding 6 patients with spontaneous abortion. A total of 59 patients were incorporated into the statistical analysis. The patients underwent three follow-up visits. During the initial visit, the participants were enrolled in the study; all of them were healthy individuals in the first trimester of pregnancy. In the second visit, an oral glucose tolerance test (OGTT) with 75 grams of glucose was conducted between 24 and 28 weeks of gestation, and the patients were categorized. Patients exhibiting a positive OGTT were diagnosed with gestational diabetes. The third visit occurred in the third trimester of gestation.Results: Gestational diabetes mellitus was registered in 14 (23.73%) patients. Body mass index had significantly higher values in the group with gestational diabetes (34.59 ± 3.9 vs 29.95 ± 5.4 kg/m2, p=0.0044). The comparison of the two groups regarding the lipid status presented significantly higher triglycerides in the group with gestational diabetes (4.01 ± 2.3 vs 2.62 ± 0.9, (p=0.0017). The other parameters of lipid status were similar between the two groups. In both groups, the changes in glucose parameters were statistically insignificant in the third, compared to the first trimester of pregnancy.Conclusion: Dyslipidemia during pregnancy is a common but complex condition with consequences for both the mother and the fetus. Key Words: gestational diabetes; glycemia; dyslipidemia; triglycerides.
Introduction
Gestational diabetes mellitus (GDM) is a form of glucose intolerance that develops during pregnancy, with a global prevalence of 17.8% (1). It results from increased insulin resistance driven by hormonal changes and adipokine activity from maternal adipose tissue (1). Women with GDM face a two- to threefold increased risk of developing type 2 diabetes later in life, while their offspring are predisposed to obesity and metabolic disorders. Risk factors include obesity, advanced maternal age, family history of diabetes, polycystic ovarian syndrome, hypertensive disorders and prior poor pregnancy outcomes (2). Obesity-associated inflammation and placental cytokines play a key role in disease onset. GDM is linked to maternal complications such as preeclampsia, gestational hypertension and cesarean delivery, and fetal risks including macrosomia, shoulder dystocia, congenital anomalies, neonatal hypoglycemia and increased NICU admissions (2,3).
Physiological changes in lipid metabolism during pregnancy lead to a progressive increase in total cholesterol, triglycerides and low-density lipoprotein cholesterol (LDL), peaking in the third trimester (4,5). While these changes support fetal development, excessive lipid levels—particularly in women with high BMI or preexisting dyslipidemia—may worsen maternal endothelial dysfunction and contribute to pregnancy complications such as preeclampsia (6–8). Despite their potential impact, lipid levels are not routinely monitored during pregnancy.
Understanding the interplay between glucose and lipid metabolism in pregnancy is crucial, particularly in high-risk populations.
Therefore, this study aims to determine whether there is a significant difference in lipid and glucose profiles between patients with gestational diabetes and healthy patients.
Material and Methods
This prospective clinical study was performed at the University Clinic for Endocrinology, Diabetes and Metabolic Diseases in Skopje, Republic of North Macedonia, for a 12-month duration, from March 2022 to March 2023. The main aim of the study was to evaluate metabolic alterations throughout pregnancy, specifically regarding the onset of gestational diabetes mellitus (GDM) and related biochemical indicators.
Study Cohort
The study initially recruited 65 clinically healthy pregnant women in their first trimester. The inclusion criteria were singleton pregnancies, no pre-existing diabetes or metabolic disorders, and consent to participate in all phases of the trial. Individuals with chronic conditions, numerous gestations, or those who did not attend follow-up appointments were omitted. From the initial cohort, six patients experienced spontaneous abortions during the study and were therefore eliminated from the final statistical analysis. Consequently, the ultimate study cohort consisted of 59 patients.
Research Methodology and Subsequent Monitoring
Participants were prospectively monitored during three clinical visits corresponding to the first, second, and third trimesters of pregnancy.
Initial Consultation (First Trimester): This appointment occurred during the early stage of gestation (gestational age ≤13 weeks). Comprehensive demographic and medical history were documented for each participant, encompassing age, gestational age, gravidity, parity and familial diabetes history. Baseline laboratory assessments were conducted, encompassing fasting plasma glucose (FPG), fasting insulin levels and serum lipid profile (triglycerides, total cholesterol, high-density lipoprotein [HDL] and low-density lipoprotein [LDL].
During the second visit, occurring between the 24th and 28th week of gestation, all participants underwent an oral glucose tolerance test (OGTT) utilizing a 75-gram glucose load, in line with the standards established by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) and the World Health Organization (WHO) in 2013. Patients were categorized into two groups based on the OGTT results: those diagnosed with gestational diabetes mellitus (GDM) and those exhibiting normal glucose tolerance (NGT).
• Third Visit (Third Trimester): The final clinical assessment occurred during the third trimester (gestational age ≥30 weeks). During this visit, laboratory tests were conducted again, encompassing fasting plasma glucose, fasting insulin levels and blood lipids (triglycerides, total cholesterol, HDL, LDL). The readings were compared to the baseline (first trimester) data to assess metabolic changes throughout pregnancy.
Data Acquisition and Biochemical Assessment
All biochemical assays were conducted in the clinic’s central laboratory utilizing standardized enzymatic and immunoassay techniques. Blood samples were collected following a minimum overnight fast of 8 hours. Glucose concentrations were assessed with the hexokinase technique. Insulin was quantified using chemiluminescent immunoassay, whereas lipid parameters were assessed by enzymatic colorimetric methods.
Statistical Analysis
The statistical analysis of the data obtained from the study was performed using the statistical program SPSS 23.0. Shapiro Wilk’s test was used to test the normality of the data distribution. The obtained data are presented in tables and graphs.
Categorical (attribute) variables are presented with absolute and relative numbers. Numerical (quantitative) variables are presented with mean, standard deviation, minimum and maximum values.
Student t-test for independent samples was used to compare the two groups.
Student t-test for dependent samples was used to test the difference in changes in the analyzed parameters in the third versus the first trimester of pregnancy.
Statistical significance was defined at the level of p<0.05.
Results
The occurrence of gestational diabetes mellitus was registered in 14 (23.73%) patients. With and without gestational diabetes were homogeneous in terms of age, i.e., patients from both groups had similar ages (31.4 ± 4.2 vs 30.0 ± 4.7 years; t=1.01 p=0.0044).
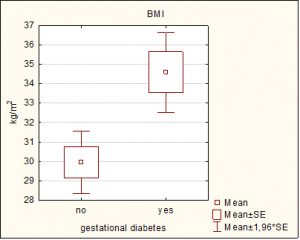
The body mass index had significantly higher values in the group with gestational diabetes (34.59 ± 3.9 vs 29.95 ± 5.4 kg/m2, p=0.0044).
Table 1. Age and body mass index of the patients with and without gestational diabetes.
| Variable | Gestational Diabetes | p-level | |
| yes
|
no
|
||
| Age (years) mean ±. SD
Min – max |
31.4 ± 4.2 24 – 39 |
30.0 ± 4.7 20 – 39 |
t=1.01 p=0.32 |
| BMI (kg/m2)
mean ± SD Min – max |
34.59 ± 3.9
29.8 – 41.1 |
29.95 ± 5.4
22.4 – 44 |
t=2.96
**p=0.0044 |
The values are shown with mean ± SD, min-max
BMI: body mass index
T (Student t-test for independent samples)
**sig p<0.01
Graph 1. Graphical presentation of average BMI in patients with/without Gestational diabetes.
Patients with and without gestational diabetes did not differ significantly in terms of glucose status: they had similar values of insulin (13.67 ± 6.9 vs 14.49 ± 9.9, p=0.77), fasting plasma glucose (4.80 ± 0.5 vs 4.54 ± 0.4, p=0.06), and HbA1c (5.22 ± 0.4 vs 5.25 ± 0.3, p=0.76).
Table 2. Insulin, glycaemia and HbA1c in patients with and without gestational diabetes.
| variable | Gestational Diabetes | p-level | |
| yes
|
no | ||
| Insulin
mean ±. SD Min – max |
13.67 ± 6.9 6.22 – 27.41 |
14.49 ± 9.9 3.63 – 51.88 |
t=0.3 p=0.77 |
| Glycaemia
mean ±. SD Min – max |
4.80 ± 0.5 4.05 – 5.8 |
4.54 ± 0.4 3.6 – 5.65 |
t=1.9 p=0.06 |
| HbA1c
mean ±. SD Min – max |
5.22 ± 0.4
4.32 – 6.12 |
5.25 ± 0.3
4.43 – 6.04 |
t=0.29
p=0.76 |
The values are shown with mean ± SD, min-max
T (Student t-test for independent samples)
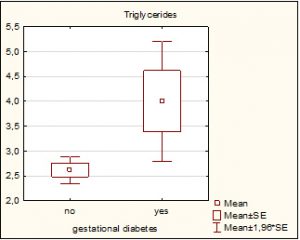
Comparison of the two groups in terms of lipid status presented significantly higher triglycerides in the group with gestational diabetes (4.01 ± 2.3 vs 2.62 ± 0.9, p=0.0017). Other lipid status parameters were similar between the two groups: total cholesterol was insignificantly higher in the group with gestational diabetes (7.13 ± 1.02 vs 7.13 ± 1.02, p=0.068); HDL had insignificantly lower values in the group with gestational diabetes (1.72 ± 0.4 vs 1.77 ± 0.4, p=0.68); LDL was insignificantly higher in the group with gestational diabetes (4.04 ± 1.1 vs 3.95 ± 0.98, p=0.3).
Table 3. Comparison of the two groups in terms of lipid status.
| variable | Gestational Diabetes | p-level | |
| yes
|
no
|
||
| Triglycerides
mean ±. SD Min – max |
4.01 ± 2.3 1.95 – 10.82 |
2.62 ± 0.9 0.98 – 5.61 |
t=3.3 **p=0.0017 |
| Total cholesterol
mean ±. SD Min – max |
7.13 ± 1.02 5.67 – 9.46 |
6.51 ± 1.1 4.36 – 9.07 |
t=1.9 p=0.06 |
| HDL
mean ±. SD Min – max |
1.72 ± 0.4 1.11 – 2.56 |
1.77 ± 0.4 0.99 – 2.7 |
t=0.4 p=0.68 |
| LDL
mean ±. SD Min – max |
4.04 ± 1.1 1.28 – 5.65 |
3.95 ± 0.98 2.34 – 6.41 |
t=0.3 p=0.8 |
The values are shown with mean ± SD, min-max
HDL: high-density lipoprotein, LDL: low-density lipoprotein.
t (Student t-test for independent samples)
**sig p<0.01
Graph 2. Graphical presentation of average triglycerides in patients with/without Gestational diabetes.
In both groups, changes in glucose parameters were statistically insignificant in the third trimester of pregnancy compared to the first trimester, while all lipid status parameters were significantly higher.
Greater changes in lipid status were registered in the group with gestational diabetes, except for the LDL parameter: the average change for triglycerides was 2.397 vs 1.34, for total cholesterol 2.17 vs 1.79, for HDL 0.27 vs 0.17.
Table 4. Change in clinical parameters.
| variable | 1 | 3 | difference | p-level | |
| GD | Insulin | 14.36 ± 5.1 | 13.67 ± 6.9 | 0.698 | t=0.3 p=0.77 |
| Glycaemia | 4.84 ± 0.6 | 4.80 ± 0.5 | 0.038 | t=0.2 p=0.86 | |
| HbA1c | 5.29 ± 0.3 | 5.22 ± 0.4 | 0.077 | t=0.8 p=0.42 | |
| Triglycerides | 1.61 ± 0.6 | 4.01 ± 2.3 | 2.397 | t=3.7 **p=0.0025 | |
| Total cholesterol | 4.96 ± 1.1 | 7.13 ± 1.0 | 2.17 | t=7.0 ***p=0.000009 | |
| HDL | 1.45 ± 0.2 | 1.72 ± 0.4 | 0.27 | t=2.2 *p=0.048 | |
| LDL | 2.98 ± 0.7 | 4.04 ± 1.1 | 1.05 | t=3.7 **p=0.0028 | |
| Weight | 86.0 ± 10.5 | 92.14 ± 11.5 | 6.14 | t=6.4 ***p=0.00002 | |
| BMI | 31.93 ± 3.7 | 34.59 ± 3.9 | 2.66 | t=4.6 ***p=0.0005 | |
| Without GD | Insulin | 12.96 ± 7.6 | 14.49 ± 9.9 | 1.53 | t=1.3 p=0.19 |
| Glycaemia | 4.68 ± 0.5 | 4.54 ± 0.4 | 0.139 | t=1.8 p=0.08 | |
| HbA1c | 5.23 ± 0.2 | 5.25 ± 0.3 | 0.026 | t=0.5 p=0.63 | |
| Triglycerides | 1.26 ± 0.8 | 2.62 ± 0.9 | 1.34 | t=9.3 ***p=0.000000 | |
| Total cholesterol | 4.71 ± 0.98 | 6.51 ± 1.1 | 1.79 | t=9.8 *** p=0.000000 | |
| HDL | 1.59 ± 0.4 | 1.77 ± 0.4 | 0.17 | t=2.9 **p=0.005 | |
| LDL | 2.73 ± 0.8 | 3.95 ± 0.98 | 1.22 | t=7.3 ***p=0.000000 | |
| Weight | 72.04 ± 15.1 | 81.0 ± 14.7 | 8.95 | t=14.4 ***p=0.000000 | |
| BMI | 26.70 ± 5.4 | 29.95 ± 5.4 | 3.24 | t=11.3 *** p=0.000000 |
The values are shown with ± SD, min-max
HDL: high-density lipoprotein, LDL: low-density lipoprotein; BMI: body mass index
t (Student t-test for dependent samples)
*sig p<0.05; **sig p<0.01; ***sig p<0.0001
Discussion
This prospective study demonstrated a correlation between glucose and lipid status in patients with gestational diabetes and healthy pregnant patients. The age at conception was comparable between the two groups, reinforcing the established understanding that, in addition to maternal age, other major risk factors for GDM include family history and obesity (9). Our findings confirmed that the body mass index was significantly higher in the group of patients with gestational diabetes compared to the group of healthy subjects. The analysis of insulin, fasting plasma glucose and HbA1c in the third trimester of pregnancy between the group of patients with gestational diabetes and the group of healthy subjects did not differ significantly in terms of glucose status: they had similar values of insulin (13.67 ± 6.9 vs 14.49 ± 9.9, p=0.77), fasting plasma glucose (4.80 ± 0.5 vs 4.54 ± 0.4, p=0.06), and HbA1c (5.22 ± 0.4 vs 5.25 ± 0.3, p=0.76). Even in the group of patients with diagnosed gestational diabetes, there is a slightly lower value of HbA1c, that may reflect the effects of early diagnosis and adherence to a prescribed regimen including dietary modifications, physical activity, and pharmacological therapy, contributing to better glycemic control.
Comparing the two groups of patients with gestational diabetes to the group of healthy participants in the third trimester of pregnancy revealed that the gestational diabetes group exhibited significantly elevated triglyceride levels. Other lipid status parameters were comparable between the two groups: total cholesterol was marginally elevated in the gestational diabetes group (7.13 ± 1.02 vs 7.13 ± 1.02, p=0.068); HDL levels were slightly reduced in the gestational diabetes group (1.72 ± 0.4 vs 1.77 ± 0.4, p=0.68); LDL was marginally higher in the gestational diabetes group (4.04 ± 1.1 vs 3.95 ± 0.98, p=0.3). The significant longitudinal increase in triglycerides and the insignificantly elevated total cholesterol and LDL in the gestational diabetes group align with the findings of Farias et al. (10), which demonstrated a linear increase in total cholesterol, LDL and triglycerides with advancing gestational weeks. Only HDL has the highest value throughout the third trimester before commencing a drop.
The study by Lippi et al. showed that gestational age markedly affected lipid parameters, with women in their second and third trimesters showing significantly elevated levels of total cholesterol, low-density lipoprotein cholesterol and triglycerides (11). Likewise, Alvarez et al. observed that triglyceride and cholesterol concentrations escalated across all lipoprotein fractions as pregnancy advanced (12).
A study was conducted to analyze the correlation of glucose parameters, including insulin, fasting plasma glucose and HbA1c, between the first and third trimesters of pregnancy. The results showed no significant difference in glucose parameters in the third trimester compared to the first trimester, while all lipid status parameters were significantly higher. The lack of significant difference in glucose parameters may be attributed to the introduced treatment, dietary changes, and increased physical activity. Greater changes in lipid status were observed in the group with gestational diabetes compared to healthy subjects, except for the LDL parameter. The average change for triglycerides was 2.397 vs 1.34, for total cholesterol 2.17 vs 1.79, and for HDL 0.27 vs 0.17. Increased body weight and obesity are serious risk factors for gestational diabetes, and patients with gestational diabetes have a higher lipid profile, especially triglycerides, compared to healthy subjects. It is essential to consider the physiological increase in total cholesterol by 25-50%, LDL by 60% and triglycerides by 200%. Therefore, overall laboratory evaluation in women before pregnancy is useful to identify patients with lipid profile disorders preconceptionally, requiring intervention with diet, physical activity and medication treatment.
Despite the availability of various treatment options for dyslipidemia in pregnant patients, only bile acid sequestrates are allowed during pregnancy. Ezetimibe and fenofibrate may be considered if the benefits outweigh the potential risks. Statins are contraindicated for use, but recent meta-analyses have shown potential benefits in strictly selected cases, especially in patients with high cardiovascular risk, recent cardiac events, established cardiovascular disease, or familial hypercholesterolemia. In these cases, the final decision should carefully consider the potential risk of discontinuing therapy. More information is needed about the treatment of dyslipidemia during pregnancy with new drugs like PCSK9 inhibitors, especially Inclisiran, which can be used before pregnancy and immediately after delivery, at intervals of 9 months between treatments. The decision to treat dyslipidemia during pregnancy should be individualized.
Conclusion
Managing dyslipidemia during pregnancy necessitates a meticulous and personalized strategy, alongside a comprehensive approach, to ensure the well-being of both mother and fetus. Physiological alterations during gestation frequently intensify lipid irregularities, especially increased triglycerides and LDL-cholesterol, potentially complicating pre-existing dyslipidemic disorders. Effective care predominantly depends on lifestyle modifications and nutritional changes, with pharmacological treatment reserved for specific high-risk situations. Inherited conditions like familial hyperchylomicronemia, necessitate individualized nutritional approaches to mitigate risks and promote positive pregnancy results.
References:
- American Diabetes Association; 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care1 January 2021; 44 (Supplement_1): S200–S210.
- Agarwal MM, Dhatt GS, Zayed R, Bali N. Gestational diabetes: relevance of diagnostic criteria and preventive strategies for Type 2 diabetes mellitus. Arch Gynecol Obstet. 2007 Sep;276(3):237-43. doi: 10.1007/s00404-007-0334-4. Epub 2007 Feb 21.
- Agarwal MM, Dhatt GS, Zayed R, Bali N. Gestational diabetes: relevance of diagnostic criteria and preventive strategies for Type 2 diabetes mellitus. Arch Gynecol Obstet. 2007 Sep;276(3):237-43. doi: 10.1007/s00404-007-0334-4. Epub 2007 Feb 21.
- Nasioudis D, Doulaveris G, Kanninen TT. Dyslipidemia in pregnancy and maternal-fetal outcome. Minerva Ginecol. 2019 Apr;71(2):155-162. doi: 10.23736/S0026-4784.18.04330-7. Epub 2018 Oct 11.
- Piechota W, Staszewski A. Reference ranges of lipids and apolipoproteins in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1992 Jun 16;45(1):27-35. doi: 10.1016/0028-2243(92)90190-a.
- Herrera E, Ortega-Senovilla H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr Pharm Biotechnol. 2014;15(1):24-31. doi: 10.2174/1389201015666140330192345.
- Barbir M, Pottle A, Bornstein SR. The implications of measuring lipoprotein(a) in clinical practice. Glob Cardiol Sci Pract. 2024 Aug 1;2024(4):e202440. doi: 10.21542/gcsp.2024.40.
- Lowe WL Jr, Karban J. Genetics, genomics and metabolomics: new insights into maternal metabolism during pregnancy. Diabet Med. 2014 Mar;31(3):254-62. doi: 10.1111/dme.12352.
- Poornima IG, Indaram M, Ross JD, Agarwala A, Wild RA. Hyperlipidemia and risk for preclampsia. J Clin Lipidol. 2022 May-Jun;16(3):253-260. doi: 10.1016/j.jacl.2022.02.005. Epub 2022 Feb 20.
- Lindsay KL, Brennan L, Rath A, Maguire OC, Smith T, McAuliffe FM. Gestational weight gain in obese pregnancy: impact on maternal and foetal metabolic parameters and birthweight. J Obstet Gynaecol. 2018 Jan;38(1):60-65. doi: 10.1080/01443615.2017.1328670. Epub 2017 Aug 6.
- Lippi G, Albiero A, Montagnana M, Salvagno GL, Scevarolli S, Franchi M, Guidi GC. Lipid and lipoprotein profile in physiological pregnancy. Clin Lab. 2007;53(3-4):173-7.
- Alvarez JJ, Montelongo A, Iglesias A, Lasunción MA, Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J Lipid Res. 1996 Feb;37(2):299-308.
- Preda A, Preda SD, Mota M, Iliescu DG, Zorila LG, Comanescu AC, Mitrea A, Clenciu D, Mota E, Vladu IM. Dyslipidemia in Pregnancy: A Systematic Review of Molecular Alterations and Clinical Implications. Biomedicines. 2024 Oct 3;12(10):2252. doi: 10.3390/biomedicines12102252.
- Vahedian-Azimi A, Bianconi V, Makvandi S, Banach M, Mohammadi SM, Pirro M, Sahebkar A. A systematic review and meta-analysis on the effects of statins on pregnancy outcomes. Atherosclerosis. 2021 Nov;336:1-11. doi: 10.1016/j.atherosclerosis.2021.09.010. Epub 2021 Sep 10.
- Lewek J, Bielecka-Dąbrowa A, Toth PP, Banach M. Dyslipidaemia management in pregnant patients: a 2024 update. Eur Heart J Open. 2024 Apr 26;4(3):oeae032. doi: 10.1093/ehjopen/oeae032. PMID: 38784103.