UDK: 616.391:577.161.2]:615.252.349.015.8:618.3
Todorova B.1, Tosheska – Trajkovska K.2, Bitoska I.1, Milenkovik T.1
1University Clinic for Endocrinology, Diabetes and Metabolic Diseases, Clinical Center “Mother Theresa” Faculty of Medicine,” Ss Cyril and Methodius” University, Skopje, Republic of North Macedonia
2Institute of Medical and Experimental Biochemistry, Faculty of Medicine,” Ss Cyril and Methodius” University, Skopje, Republic of North Macedonia
Abstract
Introduction: Vitamin D is crucial in the metabolism of calcium and phosphorus, as well as in bone health, while also contributing to numerous other bodily functions. Vitamin D deficiency in pregnant women is very common worldwide. It is associated with an increased risk of preeclampsia, gestational diabetes mellitus and cesarean section. Consequences in the newborn are most often associated with low birth weight, risk of neonatal hypocalcemia, asthma, and/ or type 1 diabetes mellitus. A lack of vitamin D during pregnancy is linked to various metabolic issues, including insulin resistance. It is considered that increased body weight has a negative effect on the concentration of vitamin D. Deficiency of 25-hydroxyvitamin D has long been considered a risk factor for glucose intolerance and most likely 1,25-dihydroxyvitamin D has a role in the regulation of insulin secretion.
Objective: To investigate how a deficiency in vitamin D impacts the onset of insulin resistance in pregnant women.
Material and Methods: A cross-sectional clinical study was conducted in the University Clinic for Endocrinology, Diabetes and Metabolic Diseases, Skopje, from March 2022 to March 2023 with 55 pregnant women in the first trimester of pregnancy. According to vitamin D values, the sample subjects were divided into three groups: a) Group 1: <20ng/ml; b) Group 2: 20-44ng/ml; and c) Group 3: >44ng/ml. We analyzed the level of insulinemia, glycemia and homeostatic model assessment for insulin resistance (HOMA IR) in the three groups.
Results: Among 55 pregnant women assessed in their first trimester, 30 (54.54%) showed a vitamin D deficiency (below 20ng/ml). Nineteen patients (34.54%) had normal vitamin D levels (ranging from 20 to 44ng/ml), while 6 (19.91%) had elevated levels (above 44ng/ml). In the group with vitamin D deficiency, the average HOMA IR value was higher at 3.14±1.59, compared to an average of 2.57±1 in the group with normal vitamin D levels.
Conclusion: A shortage of vitamin D during the first trimester of pregnancy is linked to increased insulin resistance, which can complicate metabolic health. Therefore, adequate supstituion of vitamin D during pregnancy is necessary for mother’s and offspring’s wellbeing.
Key Words: glycemia; insulin resistance; pregnancy; vitamin D.
Introduction
Vitamin D plays an essential part in regulating calcium and phosphorus metabolism and maintaining bone health, while also contributing to a wide range of other bodily processes. A lack of vitamin D is frequently observed during pregnancy and has been connected to heightened risks of complications such as preeclampsia, gestational diabetes, early delivery, cesarean section, and delivering a baby smaller than expected for its gestational age. For newborns, this deficiency is often tied to issues like low birth weight, a greater chance of neonatal hypocalcemia, asthma and the potential development of type 1 diabetes. Additionally, there appears to be a link between conditions like attention deficit disorder and autism spectrum development (1). Vitamin D is a steroid hormone that plays a role in regulating body homeostasis, including cardiovascular function. A connection exists between low vitamin D levels and a rise in cardiovascular risk factors. It’s believed that providing vitamin D supplements could enhance outcomes for individuals with heart-related conditions. Beyond its role in bone health, vitamin D exhibits anti-inflammatory properties and influences various systems in the body. It has been linked to the emergence of infectious diseases, autoimmune disorders, cardiometabolic conditions and the initiation of certain cancers (2). Insulin resistance is a multifaceted condition that contributes to the development of cardiovascular risk factors. As such, it is viewed as either a direct outcome or an indirect result of insufficient vitamin D levels (2). Vitamin D deficiency can be blamed on the same pathogenetic mechanisms that lead to the development of insulin resistance. Insulin resistance can be improved with proper diet and physical activity. Diet and exercise are believed to be linked to higher vitamin D levels, and addressing insulin resistance is key to boosting those levels. This underscores the importance of preventing vitamin D deficiency in pregnant women. At present, a vitamin D level of approximately 30ng/mL is advised during pregnancy. Vitamin D, a fat-soluble steroid prohormone, serves endocrine, autocrine and paracrine roles, and exists in forms like ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Its primary metabolites, produced through hydroxylation, include calcidiol (25(OH)D) and calcitriol (1,25-dihydroxyvitamin D3, or 1,25(OH)2D) (3).
Vitamin D acts as a signaling molecule and plays a role in regulating the transcription of about 3% of the human genome. In the bloodstream, it binds to the vitamin D-binding protein, which carries it to the liver, where it is transformed into 25(OH)D by the enzyme 25-hydroxylase. This compound is then converted in the kidneys into 1,25(OH)2D, the active form of vitamin D, through the action of 25-hydroxyvitamin D-1 alpha-hydroxylase. Additionally, vitamin D has been found to interact with the insulin receptor gene, suggesting its involvement in the transcriptional regulation of insulin (4).
Glutathione plays a vital role in managing vitamin D levels by aiding in its transformation into active metabolites. Vitamin D, in turn, boosts glutathione levels, helping to lower oxidative stress. Consequently, a lack of glutathione is tied to insulin resistance, a common feature in metabolic disorders like obesity and diabetes. In individuals with type 2 diabetes, vitamin D supports pancreatic beta-cell function, with calcitriol acting as a signaling molecule that interacts directly with beta-cell receptors to enhance their performance. It also influences insulin release by controlling calcium channel activity, improves insulin sensitivity by promoting insulin receptor expression, and activates the peroxisome proliferator receptor delta. Furthermore, vitamin D helps curb chronic inflammation by suppressing inflammatory cytokines linked to insulin resistance.
In humans, vitamin D is primarily activated through skin exposure to sunlight, consumption of foods high in vitamin D2 and D3, or supplementation. However, determining an ideal 25(OH)D concentration remains debated, with no universally agreed-upon thresholds for optimal vitamin D levels. Most of the tissues and organs possess vitamin D receptors, highlighting its involvement in numerous biological processes. During pregnancy, a deficiency in vitamin D has been connected to various metabolic issues, including insulin resistance. Pregnancy naturally increases insulin resistance, a key indicator of gestational diabetes, and excessive weight gain during this period is believed to further reduce vitamin D levels (5).
Objectives
The objectives of the study were to determine the effect of vitamin D deficiency during pregnancy on the development of insulin resistance.
Materials and Methods
A cross-sectional clinical study was conducted at the University Clinic of Endocrinology, Diabetes and Metabolic Diseases, Skopje, from March 2022 to March 2023. The study included 55 patients in the first trimester of pregnancy. A detailed medical history for each patient was used to obtain data on demographic characteristics, gestational week of pregnancy and vitamin D values, fasting insulinemia, fasting glycemia, and an insulin resistance value calculation homeostatic model assessment for insulin resistance (HOMA IR) was performed.
According to vitamin D values, the sample subjects were divided into three groups: a) Group 1: <20ng/ml; b) Group 2: 20-44ng/ml; and c) Group 3: >44ng/ml. We analyzed the level of insulinemia, glycemia and HOMA IR in the three groups.
Statistical Processing
The data was processed in the SPSS software package, version 22.0 for Windows. Qualitative series were analyzed with ratios, proportions and rates, and quantitative series with measures of central tendency and measures of dispersion. The Shapiro-Wilk W test was used to determine the regularity of the frequency distribution of the variables examined. The Pearson Chi-square test was used to determine the association between certain traits in the groups of respondents. The Pearson correlation coefficient, as well as Partial correlations were used to determine the association between the numerical variables of vitamin D and HOMA IR with no adjustment for age and gestational week of pregnancy. Independent numerical parameters were compared with the Kruskal-Wallis H test. To determine statistical significance, a two-sided analysis with a significance level of p<0.05 was used.
Results
The study enrolled 55 patients in the first trimester of pregnancy, with a median gestation week of the whole sample of 9.76 ± 1.89, with a minimum of 6 and maximum of 13 weeks of gestation, and with 50% of patients below 10 weeks of gestation for Median IQR=10 (8-11). The mean age of the patients in the sample was 30±4.66 years with a min/max age of 20/39 years. For 75% of respondents in the sample, the age was less than 34 years for a median IQR of 31 (29-34).
The mean vitamin D value in the entire sample of respondents was 22.87±14.50ng/mL, with a minimum value of 3ng/mL and 70ng/mL, respectively. In 50% of pregnant women, the vitamin D value was less than 18.12ng/mL. According to vitamin D values, the sample subjects were divided into three groups: a) Group 1: <20ng/ml in 30 (54.54%); b) Group 2: 20-44ng/ml in 19 (34.54%); and c) Group 3: >44ng/ml in 6 (19.91%). The mean vitamin D value for the three groups was consequentially 13.66±3.98 vs. 26.53±4.42 vs. 57.35±11.11ng/ml, a significant difference between the groups with the expected lowest mean value in Group 1 and highest in Group 3 (Kruskal-Wallis H test: Chi-square (2) = 42.956; p = 0.00001) (Table 1).
In pregnant women in the three vitamin D groups, the mean insulin was 14.72±7.16 in Vit D Group 1, 12.81±5.12 in Vit D Group 2, and 14.79±6.28 in Vit D Group 3. In 50% of the subjects in the three groups, insulin values were consequentially < 14.4 vs. 13.5 vs. 13.7 with no significant intergroup difference (Kruskal-Wallis H test: Chi-square (2)=0.478; p=0.7873) (Table 1).
Table 1. Intergroup comparison of vitamin D by selected parameters.
| Parameters | Values Obtained | ||||||
| (No.) | Mean± SD | (Min/Max) | Percentiles | 1p | |||
| 25th | 50th (Median) | 75th | |||||
| Vitamin D (ng/ml) | |||||||
| Vit D – Group 1 | 30 | 13.66±3.98 | 3/ 19.7 | 11.7 | 14 | 17.1 | X2(2)=42.956;
p=0.00001* |
| Vit D – Group 2 | 19 | 26.53±4.42 | 20.8/ 37.5 | 22.3 | 26 | 30.1 | |
| Vit D – Group 3 | 6 | 57.35±11.11 | 44.5/ 7 | 45.9 | 56.9 | 70 | |
| Insulin | |||||||
| Vit D – Group 1 | 30 | 14.72±7.16 | 5.2/ 40.9 | 10.1 | 14.1 | 17.9 | X2(2)=0.478;
p=0.7873 |
| Vit D – Group 2 | 19 | 12.81±5.12 | 2.4/ 20.2 | 9.3 | 13.5 | 16.4 | |
| Vit D – Group 3 | 6 | 14.79±6.28 | 7.9/ 24.7 | 9.5 | 13.7 | 19.1 | |
| Glycemia | |||||||
| Vit D – Group 1 | 30 | 4.88±0.55 | 3.4/ 5.8 | 4.6 | 5 | 5.2 | X2(2)=5.500;
p=0.0639 |
| Vit D – Group 2 | 19 | 4.59±0.40 | 4/ 5.4 | 4.2 | 4.6 | 4.9 | |
| Vit D – Group 3 | 6 | 4.88±0.31 | 4.5/ 5.3 | 4.6 | 4.9 | 5.1 | |
| HOMA IR | |||||||
| Vit D – Group 1 | 30 | 3.14±1.59 | 0.9/ 8.9 | 2.2 | 3.2 | 3.6 | X2(2)=1.726;
p=0.4220 |
| Vit D – Group 2 | 19 | 2.57±1.15 | 0.5/ 4.7 | 1.9 | 2.6 | 3.1 | |
| Vit D – Group 3 | 6 | 3.18±1.47 | 1.7/ 5.4 | 1.9 | 2.8 | 4.5 | |
| Vit D – Group 1: <20ng/ml; Vit D – Group 2: 20-44ng/ml; and Vit D – Group 3: >44ng/ml
1Kruskal-Wallis H test *significant for p<0.05 |
|||||||
Analysis in terms of glycemic levels indicated a marginally lower average glycemic value in Vitamin D-Group 2 of 4.59±0.40 compared to Vitamin D-Group 1 and Vitamin D-Group 3, where the mean value was consequentially 4.88±0.55 vs. 4.88±0.31 (Kruskal-Wallis H test: Chi-square (2) = 5.50; p = 0.0639) (Table 1).
The mean HOMA IR was indistinctly low in Vitamin D-Group 2 of 2.57±1.15 compared to Vitamin D-Group 1 and Vitamin D-Group 3, where the mean HOMA IR was consequentially 3.14±1.59 vs. 3.18±1.47 (Kruskal-Wallis H test: Chi-square (2) = 1.726; p=0.4330) (Table 1).
The majority of patients from the three Vitamin D groups had a HOMA IR≥2.5 and 22 (73.33%) in Vitamin D-Group 1; 11 (61.11%) in Vitamin D-Group 2; and 4 (66.67%) in Vitamin D-Group 3 (Table 2). No significant association was found between patients belonging to any of the three vitamin D groups and HOMA IR values <2.5 and ≥2.5 (X2=0.7898; df=2; p=0.6737).
Table 2. Analysis of vitamin D and HOMA IR groups.
| Vitamin D | HOMA IR | Total | 1p | |
| HOMA IR <2.5 | HOMA IR ≥2.5 | |||
| Groups | ||||
| Vit D – Group 1 | 8 (26.67%) | 22 (73.33%) | 30 (55.56%) | X2=0.7898; df=2;
p=0.6737 |
| Vit D – Group 2 | 7 (38.89%) | 11 (61.11%) | 18 (33.33%) | |
| Vit D – Group 3 | 2 (33.33%) | 4 (66.67%) | 6 (11.11%) | |
| Vit D – Group 1: <20ng/ml; Vit D – Group 2: 20-44ng/ml; и Vit D – Group 3: >44ng/ml
X2 = Pearson Chi-square test; *significant for p<0.05 |
||||
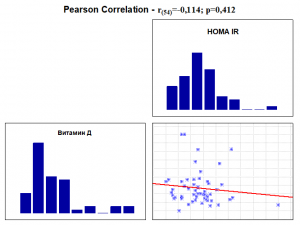
An analysis was performed on the correlation of vitamin D levels with HOMA IR levels without and with gender-adjusted gestational weeks of study pregnant women (Table 3 and Graph 1).
Table 3. Correlation between vitamin D and HOMA IR without and with adjustment for age and gestation week.
| Option | HOMA IR | ||
| unajusted1 | ajusted2 | ajusted3 | |
| Vitamin D | r (54)=-0.114; p=0.412 | r (54)=-0.795; p=0.571 | r (54)=-0.095; p=0.500 |
| 1Pearsons moment order correlations 2Partial correlations – adjusted for age;
3Partial correlations – Adjusted for gestational weeks *significant for p<0.05 |
|||
Correlation analysis revealed the presence of a nondistinct linear negative correlation between vitamin D and HOMA IR levels (r(54)=-0.114; p=0.412) with increasing vitamin D levels and nondistinctly decreasing HOMA IR levels. No significant difference was found in the strength of the correlation between vitamin D levels and HOMA IR levels before and after age-related adjustment and gestational age (Table 3 and Graph 1).
Graph 1. Correlation between Vitamin D and HOMA IR without and with Adjustment.
Discussion
In our study of the 55 patients evaluated in the first trimester, 30 (54.54%) were deficient in vitamin D (<20ng/ml). Normal vitamin D values (20–44ng/ml) were observed in 19 patients (34.54%), while higher vitamin D levels (> 44ng/ml) were found in 6 patients (19.91%). In the first group of patients with vitamin D deficiency, a higher average insulin value of 14.72 was observed compared to the average insulin value of 12.81 in the normal group of patients with vitamin D. In terms of glycemia, it indicated a marginally lower average glycemic value in Vitamin D-Group 2 of 4.59±0.40 compared to Vitamin D-Group 1 and Vitamin D-Group 3, where the mean value was consequentially 4.88±0.55 vs. 4.88±0.31.
The study by Maghbooli et al., which analyzed 741 pregnant women, found that the prevalence of vitamin D deficiency was found in 70.6% of pregnant women. The prevalence of severe vitamin D deficiency (<12.5nmol/L) in patients with gestational diabetes was higher than in patients without gestational diabetes. They found a positive correlation between vitamin D and insulin sensitivity. Vitamin D deficiency could be a sign of insulin resistance and a higher probability of gestational diabetes during the pregnancy (5).
Christoph and colleagues, in a study involving 1,382 pregnant women, reported that 73.23% of the pregnant women are with a deficit of vitamin D. A severe deficit of vitamin D (vitamin D levels below 25nmol/L) was found in 34.2% of all pregnant women. They found an association between low vitamin D, increased insulin levels and gestational diabetes (6).
In our study, the vitamin D-deficient group had a higher average HOMA IR of 3.14±1.59 compared to the normal vitamin D group with a HOMA IR of 2.57±1.15, similar to the previous cited studies.
Diseases associated with insulin resistance are becoming all too common. Vitamin D deficiency has been blamed at the molecular level as one of the risk factors leading to insulin resistance (7).
Prevention from cardiometabolic diseases, cancer development and anti-inflammatory properties are the main extra skeleton activity of vitamin D (8). Supplementation with vitamin D during pregnancy in a woman with a low level of vitamin D can improve the growth of the fetus and reduce the risks for small for gestational age, preterm birth, preeclampsia and gestational diabetes. The link between vitamin D deficiency and adverse maternal outcomes is very common, like high blood pressure during pregnancy, preterm delivery, cesarian section recurrent pregnancy loss and postpartum depression (9).
Mothers who have sufficient levels of vitamin D have offspring with less attention deficit, hyperactive disorders and autism (10). A severe deficit of vitamin D in the pregnant woman has been associated with disordered skeletal homeostasis, congenital rickets and fractures in the newborn (11).
According to the National Institute for Health and Clinical Excellence, United Kingdom, the daily dosage of Vitamin D in all pregnant women should be 400IU (12.) According to the Endocrine Society the average dose is 1500–2000 IU (13), and 2000 IU by the Canadian Society (14).
Emerging research has highlighted additional consequences of vitamin D deficiency during pregnancy that extend beyond those previously discussed. One significant area of concern is the potential impact on the epigenetic regulation of the developing fetus. Vitamin D plays a crucial role in the regulation of gene expression through epigenetic mechanisms, such as DNA methylation. Deficiencies in vitamin D during pregnancy have been associated with alterations in these epigenetic marks, which can influence fetal development, and have lasting effects on offspring health. Notably, studies have demonstrated that maternal vitamin D deficiency can lead to changes in DNA methylation patterns that persist across multiple generations, affecting both somatic and germline tissues. These epigenetic modifications have been linked to variations in body weight and metabolic function in the offspring (15, 16). Adequate vitamin D levels are essential for proper neurodevelopment. Emerging evidence suggests that maternal vitamin D deficiency may be associated with an increased risk of neurodevelopmental disorders in offspring, such as schizophrenia. Vitamin D is essential for the normal development of the nervous system, and its deficiency during pregnancy can cause prenatal neurodevelopmental defects, influencing neurotransmission and brain function (17). Vitamin D is known to modulate the immune system, and its deficiency during pregnancy may have implications for the immune development of the fetus as well. While specific studies on this aspect are limited, it is plausible that inadequate maternal vitamin D levels could influence the neonatal immune response, potentially affecting susceptibility to infections and the development of autoimmune conditions later in life (18).
Conclusion:
General screening for vitamin D deficiency, the timing of supplementation before conception, and personalized vitamin D dosing appear essential, possibly resulting in better maternal health and advantages for children. Providing vitamin D supplements to those who are deficient is essential not only to address this issue but also to improve overall pregnancy outcomes, potentially reducing related risks and supporting maternal and fetal wellbeing.
References:
- Pérez-López, Faustino R.; Pilz, Stefan ; Chedraui, Peter ; Vitamin D supplementation during pregnancy: an overview. Current Opinion in Obstetrics and Gynecology, 2020 October, 32(5):p 316-321.
- Trimarco V, Manzi MV, Mancusi C, Strisciuglio T, Fucile I, Fiordelisi A, Pilato E, Izzo R, Barbato E, Lembo M, Morisco C; Insulin Resistance and Vitamin D Deficiency: A Link Beyond the Appearances. Front Cardiovasc Med. 2022; Mar 17;9:859793.
- Contreras-Bolívar V, García-Fontana B, García-Fontana C, Muñoz-Torres M.; Mechanisms Involved in the Relationship between Vitamin D and Insulin Resistance: Impact on Clinical Practice. Nutrients. 2021 Oct 1;13(10):3491.
- Bikle DD. Vitamin D: Production, Metabolism, and Mechanisms of Action. [Updated 2021 Dec 31]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
- Maghbooli Z, Hossein-Nezhad A, Karimi F, Shafaei AR, Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab Res Rev. 2008 Jan-Feb;24(1):27-32.
- Christoph P, Challande P, Raio L, Surbek D. High prevalence of severe vitamin D deficiency during the first trimester in pregnant women in Switzerland and its potential contributions to adverse outcomes in the pregnancy. Swiss Med Wkly. 2020 May 28;150.
- Argano C, Mirarchi L, Amodeo S, Orlando V, Torres A, Corrao S. The Role of Vitamin D and Its Molecular Bases in Insulin Resistance, Diabetes, Metabolic Syndrome, and Cardiovascular Disease: State of the Art. Int J Mol Sci. 2023 Oct 23;24(20):15485.
- Szymczak-Pajor I, Śliwińska A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients. 2019 Apr 6;11(4):794.
- Mithal A, Kalra S. Vitamin D supplementation in pregnancy. Indian J Endocrinol Metab. 2014 Sep;18(5):593-6.
- Pérez-López FR, Pilz S, Chedraui P. Vitamin D supplementation during pregnancy: an overview. Curr Opin Obstet Gynecol. 2020 Oct;32(5):316-321.
- Pawley N, Bishop NJ. Prenatal and infant predictors of bone health: the influence of vitamin D. Am J Clin Nutr 2004;80:1748S–51S.
- Godel JC. Postition statement vitamin D supplementation: Recommendations for Canadian mothers and infants. [Last accessed on 2014 Mar 15].
- Marya RK, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Invest. 1981;12:155–61.
- Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecol Obstet Invest. 1987;24:38–42.
- Xue J, Gharaibeh RZ, Pietryk EW, Brouwer C, Tarantino LM, Valdar W, Ideraabdullah FY. Impact of vitamin D depletion during development on mouse sperm DNA methylation. Epigenetics. 2018;13(9):959-974. doi: 10.1080/15592294.2018.1526027. Epub 2018 Oct 21.
- Xue J, Schoenrock SA, Valdar W, Tarantino LM, Ideraabdullah FY. Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin Epigenetics. 2016 Oct 12;8:107. doi: 10.1186/s13148-016-0276-4.
- Cherniack EP, Levis S, Troen BR. Hypovitaminosis D: a widespread epidemic. Geriatrics. 2008 Apr;63(4):24-30. PMID: 18376898.
- Martens PJ, Gysemans C, Verstuyf A, Mathieu AC. Vitamin D’s Effect on Immune Function. Nutrients. 2020 Apr 28;12(5):1248. doi: 10.3390/nu12051248. PMID: 32353972; PMCID: PMC7281985.