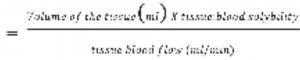UDK: 615.211.032.2:616-089.5
Mikjunovikj Derebanova Lj.1
1 University Clinic for Traumatology, Orthopedics, Anesthesiology, Resuscitation, Intensive Care and Emergency Center – Skopje, Department of Anesthesiology, Resuscitation and Intensive Care Medicine, “Ss Cyril and Methodius” University – Skopje, Faculty of Medicine
Abstract
Inhalational anesthetics are the most common drugs used for providing general anesthesia for surgery. They are used to induce unconsciousness, amnesia and immobility. In search for ideal anesthetic gas there have been discovered many agents, but sadly the perfect inhalational drug has not been produced. The pharmacokinetics of inhalational anesthetics depends on their physical properties. The rate of uptake and elimination of the inhalational anesthetics from the alveoli in mainly depends on their blood solubility. The main driving force of absorption and distribution of the gas through the body is partial pressure gradient on both sides of each barrier in the gas flow, and the therapeutic effect of the gas depends of the partial pressure of the anesthetic in the brain. All inhalational anesthetics, with exception of nitrous oxide and xenon, are metabolized (in different degrees) in liver via cytochrome P450 enzymes. The potency of different inhalational anesthetics is expressed via minimum alveolar concentration, which is defined as concentration of the anesthetics that prevent movement in 50% of the patients in response to surgical incision under standard conditions. Nitrous oxide and volatile halogenated ethers (desflurane, isoflurane, sevoflurane) are examples of medical gases that are greenhouse gases with great impact to global warming. This article summarizes a brief historical timeline of the inhalational anesthetics, mechanisms of action, physical characteristics and pharmacokinetic properties of inhalational agents with a short overview of their toxicity and pollution of the environment.
Keywords: FA/FI ratio, greenhouse gas, inhalational anesthetics, minimum alveolar concentration, pharmacokinetics, solubility.
Historical Prospective
Inhalational anesthetics were discovered way before the induction of intravenous anesthetic drugs. Since 1840’s, there has been continuous search for ideal gas. The discovery of inhalational agents with some of their characteristics is presented in the following timeline:
- In 1842, dentist Horace Wells for the first time used nitrous oxide on himself for pain-relief. Two years later, he publicly demonstrated painless dental surgery using nitrous oxide which was not completely successful, and he was discredited. Nitrous oxide is colorless gas with sweet odor and taste, but also weakest general anesthetic.
- In 1842, Crawford Long administered diethyl ether to a patient (1). Four years later, on October 16th, 1846, Boston dentist William Morton publicly demonstrated ether’s anesthetic properties. This day is now commemorated as “Ether Day”. Diethyl ether is a highly flammable colorless volatile liquid with hash side effects like nausea and vomiting.
- Chloroform was introduced in 1847 by obstetrician from Edinburgh, James Simpson, as nonexplosive alternative to ether. It is colorless with sweet smelling, dense liquor, but due to several unexplained intraoperative deaths and numerous cases of hepatotoxicity, chloroform was stopped for usage.
- The modern era of volatile anesthetics began with the discovery of halothane in 1951 by Suckling, and in 1956 it was introduced into clinical practice. The same year when halothane was discovered, xenon was for the first time used as a surgical anesthetic by American anesthesiologist Stuart C. Cullen (2). Halothane is colorless, potent volatile anesthetic with sweet smell and unstable on light. Due to unpredictable liver damage and his dysrhythmogenic effects on myocardium, the search for better volatile anesthetic continued.
- Methoxyflurane was introduced in 1960 into clinical practice. It is a colorless liquid with a fruity odor. Although, it is extremely potent, it has slow onset and offset times. Few years after its introduction, dose-related nephrotoxicity was confirmed with methoxyflurane anesthesia.
- In 1973, enflurane was introduced in clinical practice. It has the same characteristics as halothane (colorless liquid with sweet odor, sensitive on light and fast induction) but without its side effects. Enflurane can cause dose-related seizures.
- Isoflurane was introduced into clinical practice in 1981, as a colorless, nonflammable liquid with pungent odor.
- In 1992 desflurane was discovered and two years later, sevoflurane. Desflurane is a colorless, nonflammable liquid with a pungent odor. It has fast induction, low potency but is expensive. Sevoflurane is a colorless, nonflammable liquid with pleasant odor. Its induction is slower than with desflurane.
Mechanism of Action
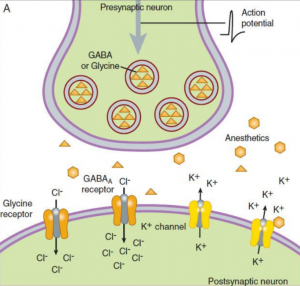
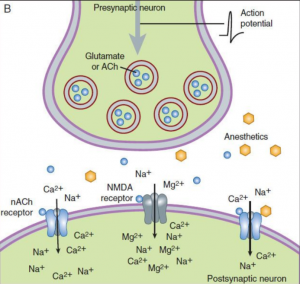
All volatile anesthetics have the same mechanism of action, but the exact mechanism is still unknown. First, Meyer (1899) and Overton (1901) developed a theory in which they believed that anesthetics bind to the bilayer lipid membrane and that their potency correlates to their solubility in lipids (3). Their theory explains that the anesthetic agent molecules bond to target sites on the lipid layer after an anesthetic agent reaches a critical level in a lipid layer. This process causes dissolution of the lipid layer of the brain cells, and the brain reaches an anesthetized state or unconscious. Later in the 1970’s, researchers demonstrated that anesthetics did not need lipid target sites for binding and that the primary site of action for anesthetics, including the inhalational anesthetics, involve proteins (4). At the end of the last century, many studies showed that the main target for inhaled anesthetics most likely are ligand gated ion channels proteins (GABA receptors, glycine, nicotinic acetylcholine, NMDA) (5-7). Physiological function of GABA and glycine receptors is to inhibit the postsynaptic excitation, so volatile anesthetics sensitized these receptors and prolong the inhibition (8, 9). Inhaled anesthetics not only have postsynaptic effect, but they also have presynaptic effect by blocking Na+ channels (NMDA receptors) and inhibited the presynaptic excitatory neurotransmitter release (10) (Figure 1). Amnesia occurs probable due to impact of inhalational anesthetics to nicotinic acetylcholine receptors (11).
Figure 1. Mechanism of action of inhalational anesthetics. A – inhibitory synapse: Inhalational anestetic enhence the binding of GABA and glycine for the GABAA receptor and increase Cl– influx causing hyperpolarized cell difficult to depolarize (↓ excitability). B – excitatory synapse: Inhalational anesthetics block Na+ channels (NMDA receptors) and inhibit the presynaptic excitatory neurotransmitter release. (Source: Helge Eilers and Spencer Yost. General anesthetics. Basicmedical Key -https://basicmedicalkey.com/general-anesthetics)(12).
GABA receptors have an inhibitory role in the adult brain, whereas, in growing developing brain GABA receptors are the main excitatory neurotransmitters. In a young child’s brain, GABA receptor opens calcium channels and increases calcium influx in the cell causing cell apoptosis (see Figure 1B). There is concern of using inhalational anesthetics in the youngest patients because, due to their exposure to inhalational anesthesia, it can cause a lasting deficit in behavior, learning and memory (13).
Physical Properties
The main physical characteristics of the inhalational anesthetics are shown in Table 1.
Table 1. Physical characteristics of inhalational anesthetics.
| Halothane | Enflurane | Isoflurane | Sevoflurane | Desflurane | N2O | Xenon | |
| Boiling point at 1 atm | 50.2 | 56.5 | 48.5 | 58.5 | 22.8 | -88.5 | -108.1 |
| Vapor pressure at 20°C | 243 | 172 | 240 | 160 | 669 | 39000 | – |
| MAC in 30-60 years, at 37°C | 0.75 | 1.7 | 1.2 | 2 | 6 | 104 | 60-70 |
| Blood: gas solubility at 37°C | 2.5 | 1.8 | 1.4 | 0.65 | 0.45 | 0.47 | 0.14 |
| Brain: blood solubility | 1.9 | 1.4 | 1.6 | 1.7 | 1.3 | 1.1 | |
| Fat: blood solubility | 51.1 | 36 | 44.9 | 47.5 | 27.2 | 2.3 | |
| Muscle: blood solubility | 3.4 | 1.7 | 2.9 | 3.1 | 2.0 | 1.2 | |
| Recovered as metabolites (%) | 20-40 | 2.4 | 0.2 | 2-5 | 0.02 | ||
| Metabolites | TFA* | TFA* | HFIP* | TFA* | – | – | |
| Preservative | Thymol | No | No | No | No | ||
| Stable in moist CO2 absorber | No | Yes | Yes | No | Yes |
*Trifluoroacetate (TFA), Hexafluoroisopropanol (HFIP)
Vapor pressure is the partial pressure exerted by the vapor in equilibrium with its liquid phase. On this pressure, equal parts of the liquid phase evaporate into gaseous phase and equal parts of gas condensate into liquor.
The boiling point is the temperature at which the liquor turns into gas or the temperature at which the vapor pressure equals to the surrounding atmospheric pressure. If the atmospheric pressure is low, like at higher altitudes, the boiling point decreases because there’s less pressure holding the liquid together. Desflurane’s boiling point of 23.5°C is near to room temperatures. It is stored in a special container (under vapor pressure) in order to escape boiling at room temperature.
According to Dalton’s law, total pressure in mixture of gases is equal to the sum of the partial pressures of each individual gas in the mixture. Mathematically, it’s expressed as:
Ptotal=P1+P2+P3+⋯
where Ptotal is the total pressure of the gas mixture and P1, P2, P3 are the partial pressures of each gas. Each gas in a mixture acts independently, which means that partial pressure of one gas is the pressure it would exert alone in the container at the same temperature and volume. Inhalational anesthetic partial pressures are expressed as volume percent (vol%) indicating the percent of the total volume contributed by a specific gas.
The partial pressure of a gas in solution refers to the pressure that the gas would exert if it were alone in a container at the same temperature. Henry’s law describes how gases dissolve in liquids based on their partial pressure. According to this law, the concentration of a gas in a liquid is directly proportional to the partial pressure of that gas above the liquid. The higher the pressure, the more gas dissolves into the liquid.
C = k x P
where C is concentration of the gas in the liquid (mol/L), k is Henry’s law constant (solubility constant specific to each gas-liquid pair), and P is partial pressure of the gas above the liquid (atm).
It is important to talk of partial pressures, because gases equilibrate based on partial pressures, not on concentrations. Inspired concentration or fractional concentration of inspired anesthetic is used as terminology rather than partial pressure.
F = Panesthetic/Pbarometric
where F is fractional concentration of anesthetic, Panesthetic is partial pressure of anesthetic. This equation shows that the fractional concentration of anesthetic is directly proportional to its partial pressure.
Pharmacokinetics of Inhalational Anesthetics
Pharmacokinetics studies how the body reacts to an administered drug during the entire time while is exposed to it. Pharmacokinetics of the inhalational agent focuses on four processes:
- Absorption or uptake (wash in) – process where the inhaled volatile agent is transported from the lung to the bloodstream,
- Distribution or tissue uptake – process where the inhaled volatile agent is transported from the bloodstream to the tissue (mainly to the effective tissue or the brain),
- Metabolism – process where the inhaled agent breaks down in the body, often in the liver, into substances that can be more easily eliminated,
- Elimination or wash out – process where the inhaled agent and its metabolites are removed from the body, through the lungs or through urine and feces.
The main driving force of absorption and distribution of the gas through the body is partial pressure gradient on both sides of each barrier in the gas flow, and the therapeutic effect of the gas depends of the partial pressure of the anesthetic in the brain.
PA (alveolar partial pressure) ↔ Pa (arterial partial pressure) ↔ Pbrain (brain partial pressure)
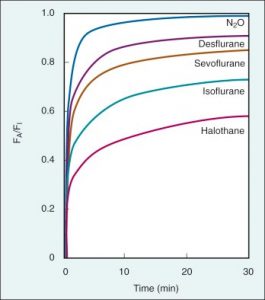
Equilibration of the partial pressures of alveolar and inspired agent is also known as “Wash in” (FA/FI) and this rate can rise from 0 to 1 (Figure 2).
Figure 2. “Wash in” of different inhalational agents depending on their solubility in patients with same CO and minute ventilation. (Modified from Yasuda N, Lockhart SH, Eger EI 2nd, et al. Comparison of kinetics of sevoflurane and isoflurane in humans. Anesth Analg. 1991; 72:316–324) (14).
The rate of rise of FA/FI depends on the rate of delivery of anesthetic to the lungs and from the rate of uptake of anesthetic from the lungs to the bloodstream. There are six factors (listed in Table 2) that determine the wash-in of the inhaled anesthetics.
Table 2. Determinants of the Wash In.
| Inspired concentration | Delivery of anesthetics TO the lungs |
| Alveolar ventilation | |
| Functional residual capacity | |
| Cardiac output | Delivery of anesthetics FROM the lungs to the blood |
| Solubility | |
| Alveolar-venous partial pressure gradient |
Increasing the inspired concentration of the anesthetic leaving the anesthesia machine by setting the vaporizer and the fresh gas flow, will increase its alveolar concentration and its rate of rise (FA/FI). This effect is also known as concentration effect.
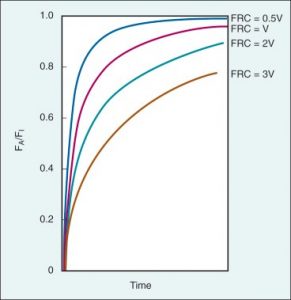
The ratio of alveolar ventilation (Va) to functional residual capacity (FRC) is the main determinant for the delivery of anesthetics to the lungs, especially for the anesthetics that are more soluble. Va/FRC ratio is one of the differences between the rate of induction in adults and neonates. In neonates this ratio is 5:1, whereas in adults is 1.5:1 (higher ratio – faster rate of rise of FA/FI). The functional residual capacity is part of the breathing circuit and greater FRC means greater volume will be needed to be saturated with gas and more time for reaching equilibration between FA and FI (Figure 3).
Figure 3. Correlation between different FRCs and the wash-in (FA/FI) in patients with the same cardiac output and minute ventilation. (Source: Pharmacokinetics of Inhaled Anesthetics. Anesthesia Key – https://aneskey.com/pharmacokinetics-of-inhaled-anesthetics) (15).
For example, obese patients have lower FRC and the induction with inhalational anesthetics will be faster.
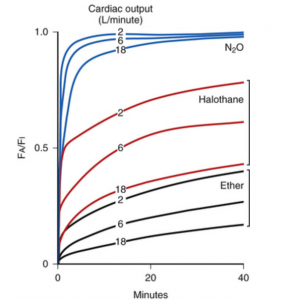
The changes in cardiac output (CO) are inversely related to the rate of rise of FA/FI (Figure 4). Patients with lower CO, like patients with heart failure, have lower blood flow through the lungs and the extraction of the gas from the alveoli into the blood is lower, so the rate of decrease of FA will be slower. This will lead to a faster rate of rise of FA/FI and a faster induction. In contrast, patients with high CO (anxiety), have faster uptake of anesthetic from the alveoli into the bloodstream, so the decrease of FA is faster, and the rate of rise of FA/FI is slower.
Figure 4. Correlation between CO and wash in rate in inhalational anesthetics with different solubility. (Source: Eger EI 2nd. Anesthetic Uptake and Action. Baltimore: Williams & Wilkins; 1974) (16).
As mentioned before, partial pressure gradient of the gas between two phases is the driving force for anesthetics to move from the alveoli to the bloodstream and from the blood to the tissue. In the moment of equilibration of these partial pressures, the partial pressure of the anesthetic in the venous blood that returns to the heart will become equal to the partial pressure of the gas in alveoli. At that moment alveolar – venous partial pressure gradient is diminished and the uptake of the gas from the alveoli stops.
The solubility of anesthetics in blood and different tissues are expressed as partition coefficients, ratio of the anesthetic distributed between two phases when the partial pressures are equal. It actually defines the affinity of the anesthetic for one particular tissue (Table 3).
Table 3. Different partition coefficients at temperature of 37°C for different inhalational anesthetics.
| Blood: Gas | Brain: Blood | Fat: Blood | |
| Nitrous oxide | 0.47 | 1.1 | 2.3 |
| Halothane | 2.5 | 2.9 | 60 |
| Methoxyflurane | 12 | 2 | 49 |
| Enflurane | 1.9 | 1.5 | 36 |
| Isoflurane | 1.4 | 2.6 | 45 |
| Desflurane | 0.45 | 1.3 | 27 |
| Sevoflurane | 0.65 | 1.7 | 48 |
A blood: gas partition coefficient of 0.65 for sevoflurane means that when the partial pressures of sevoflurane are in equilibrium, the concentration of sevoflurane in the alveolus is 1 and 0.65 in blood. This means that sevoflurane has low solubility in blood which leads to fast induction and recovery from anesthesia. In this case, the blood reservoir is small, and the anesthetic can pass into/out of the brain quicker. While, for halothane blood: gas partition coefficient is 2.5 and means that when the partial pressures of halothane are in equilibrium, the concentration in the alveolus is 1 and 2.5 in blood. Blood acts as a reservoir (store) for the drug, so it does not enter or leave the brain until the blood reservoir is filled with drug. Halothane has high solubility in blood which leads to slow induction and recovery. As you can see from Figure 2 and Table 3, nitrous oxide has the fastest rate of rise od FA/FI although desflurane has lower solubility (0.45 vs 0.47 for N2O). That is due to the concentration effect (we use 50-70% inspired concentration of N2O compared to 6% desflurane).
High solubility does not mean high potency. The potency of the inhalational anesthetics is expressed through minimal alveolar concentration (MAC). MAC is defined as concentration of the anesthetics which prevent movement in 50% of the patients in response to surgical incision under standard conditions (atmospheric pressure and room temperature). MAC is analogous to ED50 in intravenous drugs and is inversely proportional to the potency (lower MAC = higher potency) (Table 4).
Table 4. Inhalational anesthetics and their MAC values.
| Agent | MAC | Potency |
| Methoxyflurane | 0.16% | The most potent |
| Halothane | 0.74% | |
| Isoflurane | 1.17% | |
| Enflurane | 1.7% | |
| Sevoflurane | 2.05% | |
| Desflurane | 6.0% | |
| Nitrous oxide | 104% | The least potent |
MAC values are additive between different inhalational agents. That means if we want to achieve 1MAC of sevoflurane, we can use 0.5 MAC nitrous oxide and 0.5 MAC sevoflurane. But this is not the case in children. 60% Nitrous oxide combined with sevoflurane or desflurane in children, decreases the MAC of sevoflurane only 20% and 26% for desflurane (17, 18).
MAC 0.3-0.4 = MAC awake is defined as concentration of the inhaled anesthetic on which response to the verbal commands are lost in 50% of the patients (awakening from anesthesia in absence of other agents). On this level amnesia occurs. MAC 1.3 is level on which immobility is achieved in 95% of the patients and is analogous to ED95 in intravenous drugs. MAC is inversely related to lipid solubility, that is, if the lipid solubility decreases, the potency decreases and MAC increases.
The distribution or tissue uptake is managed by the same factors as the uptake of the anesthetic from the alveoli to the blood. These factors are tissue blood flow, tissue solubility (tissue: blood partition coefficient) and arterial blood-tissue partial pressure gradient. Tissues are classified in four groups according to their blood flow: vessel-rich group (VRG) (brain, heart, kidney, lung, liver), lean group (muscle and skin), vessel-poor group (connective tissue, bones) and fat (Table 5).
Table 5. Tissue classification according to their blood flow.
| Body mass (%) | CO(%) | |
| Vessel-rich group | 10 | 75 |
| lean group | 50 | 20 |
| vessel-poor group | 20 | <1 |
| fat | 20 | 5 |
Since vessel-poor group receives small percentage of cardiac output, the distribution and equilibration of the inhalational anesthetic is happening in only three groups (VRG, muscle and fat). Equilibration happens when the anesthetic partial pressures in blood and tissues approaches that to the alveoli. The rate (time) at which this equilibration takes place is expressed as time constant (𝛕).
1 𝛕 is the time for 63-67% equilibration of partial pressures between tissue and the blood and for 98% equilibration is needed 3 – 4 times constants. Knowing that VRG receives 75% of cardiac output, time constant for this group (including the brain) is short and the equilibration is the fastest. Time constant for fat is very slow due to the high fat: blood partition coefficients of the different anesthetics (see Table 1). Only nitrous oxide has similar partition coefficients in all phases. More soluble agents have longer time constant. In order fat to be saturated with the gas, more time is needed to equilibration to be achieved (anesthesia should least more than 4 hours).
All inhalational anesthetics, with exception of nitrous oxide and xenon, are metabolized in liver in different degrees. They are metabolized via cytochrome P450 enzymes in the liver, mainly by CYP 2E1 (19). Halothane (up to 40% of absorbed dose), isoflurane (0.2%) and desflurane (0.02% of absorbed dose) are bio-transformed to trifluoroacetate (TFA). TFA acts as a hapten and binds covalently to hepatocyte proteins causing hepatic injury (20). Sevoflurane is metabolized 2 – 5% in the liver and its main metabolite is hexafluoroisopropanol which does not have same antigenic characteristics as TFA. The inhalational anesthetics that undergo little metabolism have become more popular while those that are metabolized in larger percentage (halothane, methoxyflurane – 75% of absorbed dose) have become a past. Most of the anesthetics are eliminated via exhalation (wash out) through the lungs.
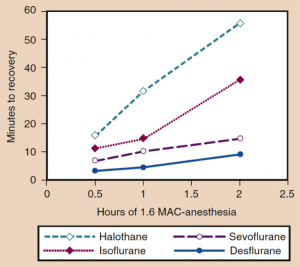
During the emergency, the inspired concentration of inhalational anesthetic is set to zero and the wash-out of inhalational anesthetics follows an exponential decay. The time of the wash-out (and speed of emergence) of the inhalational anesthetics, depends on the duration of anesthesia and solubility of the anesthetic (14). Due to their blood solubility, the speed of emergency follows the order: desflurane > sevoflurane > isoflurane > halothane > methoxyflurane, especially if the fixed MAC is maintained till the end of the surgery (18). Also, the speed of emergency in parallel depends on the duration of anesthesia. Differences between various inhalational agents are less if the duration of anesthesia is short (Figure 5) (21).
Figure 5. The time of recovery increases parallel to the duration of the anesthesia (Source: Eger EI II. Desflurane (Suprane): A Compendium and Reference. Nutley, NJ: Anaquest; 1993:1-11) (22).
There are a few strategies that can be used to speed the recovery from anesthesia. Discontinuing nitrous oxide accelerates the wash-out of inhalational anesthesia due to the second gas effect that will be mentioned later (23). Charcoal filters have been shown that can speed the emergence by absorbing the anesthetics from the anesthesia breathing circuits (24). Hypercapnic hyperventilation together with charcoal filter has been shown to speed recovery from isoflurane, sevoflurane and desflurane anesthesia by near 60% (25). Related data for children are missing.
Special Factors
Two types of shunts occur: left-to-right, and right-to-left shunt. A left-to-right shunt happens in conditions where the blood from the heart recirculates into the lungs due to some intracardiac defect. This shunt has clinical significance for pharmacokinetics of the intravenous drugs and does not affect the PK of the inhalational drugs. A right-to-left shunt happens when the venous blood bypasses the lungs and returns to the heart. These conditions can be with intracardiac etiology (due to cyanotic heart disease) or intrapulmonary etiology (due to pneumonia or endobronchial intubation). A right-to-left shunt decreases the rate of wash in, especially for the less soluble anesthetics (sevoflurane, desflurane), so the induction is slower (26, 27). In these circumstances, when inhalational anesthetics are used for induction, intravenous anesthetics are required in order to achieve satisfactory depth of anesthesia.
There are a few factors that affect the faster wash-in (induction) in infants and children compared to adults. Infants have a greater ratio of alveolar ventilation to functional residual capacity (Va/FRC), which is 5:1 vs 1.5:1 in adults. A greater fraction of cardiac output is delivered to the vessel-rich group tissues in infants. Vessel – rich group constitutes 18% of the body weight in infants compared to 10% in adults. Infants have lower solubility in blood (lower tissue: blood and blood: gas partition coefficients) than in adults, due to the lower serum cholesterol and protein levels. But blood solubility of less soluble inhalational anesthetics such as sevoflurane, is similar in infants and adults (28).
Chemical Degradation
Sevoflurane undergoes chemical degradation in carbon dioxide absorbents to produce vinyl ether compound A. The production is greater in closed circuit breathing system, low flow and by warm and dry CO2 absorbents (29). Barium hydroxide lime produces more compound A, compared to soda lime due to higher absorbent temperature during CO2 extraction (30).
Compound A causes renal tubular necrosis in rats (31). Several studies have shown that sevoflurane in closed system with low flow produces compound A nearly 8 to 24ppm and 20 to 32ppm with soda lime and barium hydroxide lime, respectively (32, 33). Prospective, multicenter, randomized study conducted in 2002, in patients with previous renal disease, showed that there were no adverse renal effects of long duration, low-flow sevoflurane (34). Most of the countries that have approved sevoflurane for clinical use have no flow restriction, perhaps because of the proven safety of sevoflurane in scientific studies. Doses of compound A up to 400ppm per hour have no toxically renal effect, even in low flow anesthesia (0.5-1L/min) (35). This nephrotoxicity is dependent on species.
Interaction between volatile anesthetics and dry CO2 absorbents can produce carbon monoxide. Factors that increase CO production include increased temperature and lower fresh gas flow, higher dryness of the absorbent and higher anesthetic concentration. Generally, temperatures in CO2 canister are 25°C to 45°C, but can be higher when using a low fresh gas flow. Significant CO production with sevoflurane is noted if the canister temperature exceeds 80°C due to exothermic reaction (36). Desflurane and isoflurane conducted with CO2 absorbents maintained at room temperature, 1 MAC desflurane produced up to 8,000ppm of CO versus 79ppm with nearly 2 MAC sevoflurane (37). Also, desflurane conducted with barium hydroxide has 3 folds higher CO production than conducted with soda lime. CO poisoning is difficult to be diagnosed because it is masked by anesthesia and pulse oximetry is unchanged (carboxyhemoglobin can’t be detected by the pulse oximetry). Carboxyhemoglobin level can range up to 40%. The production of carbon monoxide is minor with sevoflurane and halothane, intermediate with isoflurane and maximum with desflurane and enflurane.
Modern CO2 absorbents, based on lithium hydroxide, have been discovered in order to minimize production of compound A and carbon monoxide. Although, these absorbents are expensive, they can be used much longer than the previous absorbents.
N2O: Concentration Effect, Second Gas Effect, Diffusion Hypoxia and Greenhouse Effect
As mentioned before, increasing the concentration of anesthetics in the inspired fraction (FI) increases the concentration of anesthetics in the alveoli (FA), and alveolar concentration faster approaches to the inspired concentration. This is known as concentration effect and has clinical relevance only for agents administrated at high concentrations (nitrous oxide and xenon). Nitrous oxide, administrated in high concentration, is quickly taken up into the bloodstream. The absorbed N2O is substituted with proportional volume of gas which leads to faster rise of FA (fractional concentration of anesthetic in alveoli) of nitrous oxide.
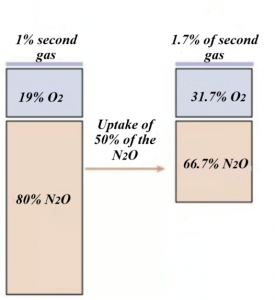
Combining N2O with the second more potent gas on induction, can increase the wash-in of the second inhalational anesthetic. This effect is called the second gas effect. For example, during induction 1% of inhalational anesthetic is delivered in 80% N2O and 19% oxygen. N2O, due to high partial pressure and low solubility, is delivered into the blood more rapidly than the other inhalational anesthetic, and the alveolar N2O concentration is decreased (e.g., by 50%). So, the uptake of N2O is 40 parts (50% from 80% in the inspired fraction of N2O), leaving 40 parts N2O, 19 parts O2, and 1% second gas in the alveoli. The second gas now in the alveoli is present at a concentration of 1/ (1 + 40 + 19) = 1.7%, and oxygen is present 19/ (1+40=19) = 31.6% (Figure 6). The second gas (potent inhalational anesthetic) has been concentrated, FA is increased and rate of rise of FA/FI is faster, which speeds the induction.
Figure 6. The second gas effect (Modified from: Pharmacology Mentor. Available from: https://pharmacologymentor.com/inhalation-anesthetics) (38).
During the emergency, when N2O is discontinued, it diffuses from blood into the alveoli very rapidly diluting alveolar oxygen. This can cause diffusion hypoxia. It can be avoided by increasing the inspired fraction of oxygen to 100% during the initial recovery.
Blood: gas partition coefficient of nitrous oxide is 0.47, whereas nitrogen blood: gas partition coefficient is 0.015, which means N2O is 30 times more soluble in blood than nitrogen. Hence, nitrous oxide diffuses more rapidly in spaces with gas that contain nitrogen than nitrogen diffuses out of them. Closed air spaces like middle ear, pneumothorax, bowel, air emboli and tracheal tube cuff can be distended due to the diffusion of N2O in them. This distention is time dependent but can be very dangerous in presence of air emboli, causing life-threatening air embolism.
All inhalational anesthetics contribute to global warming, except xenon which is an inert gas. Isoflurane, desflurane and sevoflurane are metabolized in very small percentage (0.2%, 0.02% and 2%, respectively) and after exhalation these agents remain in form that can pollute the environment. These are very powerful greenhouse gases, which can trap radiation and heat in the atmosphere until they undergo degradation in the atmosphere. The atmosphere lifetime varies between different inhalational agents: desflurane 14 years, isoflurane 3 years, sevoflurane 2 years and nitrous oxide can stay in the atmosphere for 114 years (39, 40).
Global warming potential (GWP) is measure of how much a greenhouse gas contributes to global warming over a period of 100 years, compared to equivalent mass of carbon dioxide (GWP of CO2 is one). The highest GWP100 has desflurane and is 2540, followed by isoflurane which value is 539, then nitrous oxide has GWP100 = 273 and sevoflurane is 144 times more potent than carbon dioxide as greenhouse gas (39). These factors are routinely updated as atmospheric chemistry is continuously changing. But we often overlook clinical potency when we think about GWP and the greenhouse emission. Desflurane has the lowest clinical potency (MAC 6.0%) of the volatile drugs, which means that to achieve an equivalent clinical effect at similar fresh gas flow rates as sevoflurane (MAC 2.0%) or isoflurane (MAC 1.2%), it requires three-to-five times higher concentration than sevoflurane or isoflurane, respectively. Although N2O has a lower GWP100 than isoflurane (273 vs 539), it is usually delivered at a concentration of 50-70%, resulting in a higher overall environmental effect (40). According to the research done by Global Carbon Project, from 1980 to 2020, N2O emission rose by 40% (41). Also, N2O is the most anesthetic agent responsible for ozone depletion.
In order to protect our environment, scavenging systems must be used to prevent waste gas accumulation (WGA). The American Society of Anesthesiologists recommends several strategies in order to reduce these greenhouse gases emissions, including the following: consider total intravenous anesthesia and regional anesthesia or at least low flow anesthesia (use low fresh gas flow), avoid desflurane and nitrous oxide (high impact inhalational anesthetics), use portable tanks of nitrous oxide that remain closed between use instead centralized N2O piping and investing in WGA trapping or WGA destroying technology (42).
References:
- Long C.W. An account of the first use of sulphuric ether by inhalation as an anesthetic in surgical operations. South Med Surg J; 1849.
- Marx T, Schmidt M, Schirmer U, Reinelt H. Xenon anesthesia. Journal of the Royal Society of Medicine. 2000; 93 (10): 513–517.
- Eger EI 2nd. The pharmacology of inhaled anesthetics. J Crit Care. 2005; 24 (2):89–100.
- Franks NP, Lieb WR. Where do general anesthetics act? Nature. 1978; 274 (5669):339–342.
- Frazer MJ, Lynch C 3rd. Halothane and isoflurane effects on Ca2+ fluxes of isolated myocardial sarcoplasmic reticulum. Anesthesiology. 1992;77(2):316–323.
- Narahashi T, Aistrup GL, Lindstrom JM, et al. Ion channel modulation as the basis for general anesthesia. Toxicol Lett. 1998;100-101:185–191.
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367(6464):607–614.
- Zimmerman SA, Jones MV, Harrison NL. Potentiation of gamma-aminobutyric acid A receptor Cl- current correlates with in vivo anesthetic potency. J Pharmacol Exp Ther. 1994;270(3):987–991.
- Yamakura T, Harris RA. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology. 2000;93(4):1095–1101.
- Nishikawa K, MacIver MB. Excitatory synaptic transmission mediated by NMDA receptors is more sensitive to isoflurane than are non-NMDA receptor-mediated responses. Anesthesiology. 2000;92(1):228–236.
- Cordero-Erausquin M, Marubio LM, Klink R, Changeux JP. Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol Sci. 2000;21(6):211–217.
- Helge Eilers and Spencer Yost. General anesthetics. Basicmedical Key -https://basicmedicalkey.com/general-anesthetics.
- Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population based cohort. Anesthesiology. 2009;110(4):796–804.
- Yasuda N, Lockhart SH, Eger EI 2nd, et al. Comparison of kinetics of sevoflurane and isoflurane in humans. Anesth Analg. 1991; 72:316–324.
- Pharmacokinetics of Inhaled Anesthetics. Anesthesia Key – https://aneskey.com/pharmacokinetics-of-inhaled-anesthetics.
- Eger EI 2nd. Anesthetic Uptake and Action. Baltimore: Williams & Wilkins; 1974.
- Taylor RH, Lerman J. Minimum alveolar concentration of desflurane and hemodynamic responses in neonates, infants, and children. Anesthesiology. 1991;75(6):975–979.
- Lerman J, Sikich N, Kleinman S, Yentis S. The pharmacology of sevoflurane in infants and children. Anesthesiology. 1994; 80(4):814–824.
- Kharasch ED, Thummel KE. Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology. 1993; 9:795–807.
- Restrepo JG, Garcia-Martín E, Martínez C, et al. Polymorphic drug metabolism in anaesthesia. Curr Drug Metab. 2009; 10:236–246.
- Sakata DJ, Gopalakrishnan NA, Orr JA, et al. Hypercapnic hyperventilation shortens emergence time from isoflurane anesthesia. Anesth Analg. 2007;104(3):587–591.
- Eger EI II. Desflurane (Suprane): A Compendium and Reference. Nutley, NJ: Anaquest; 1993:1-11.
- Peyton PJ, Chao I, Weinberg L, et al. Nitrous oxide diffusion and the second gas effect on emergence from anesthesia. Anesthesiology. 2011; 114(3):596–602.
- Chang DJ, Choi SH, Choi YS, Min KT. Effect of charcoal filter on the emergence from sevoflurane anesthesia in a semi-closed rebreathing circuit. Yonsei Med J. 2011; 52(4):668–672.
- Sakata DJ, Gopalakrishnan NA, Orr JA, et al. Hypercapnic hyperventilation shortens emergence time from isoflurane anesthesia. Anesth Analg. 2007;104(3):587–591.
- Tanner GE, Angers DG, Barash PG, et al. Effect of left-to-right, mixed left-to-right, and right-to-left shunts on inhalational anesthetic induction in children: a computer model. Anesth Analg. 1985; 64(2):101–107.
- Huntington JH, Malviya S, Voepel-Lewis T, et al. The effect of a right-to-left intracardiac shunt on the rate of rise of arterial and end-tidal halothane in children. Anesth Analg. 1999; 88(4):759–762.
- Malviya S, Lerman J. The blood/gas solubilities of sevoflurane, isoflurane, halothane, and serum constituent concentrations in neonates and adults. Anesthesiology. 1990; 72(5):793–796.
- Fang ZX, Kandel L, Laster MJ, et al. Factors affecting production of compound A from the interaction of sevoflurane with Baralyme and soda lime. Anesth Analg. 1996; 82:775–781.
- Frink EJ Jr, Malan TP, Morgan SE, et al. Quantification of the degradation products of sevoflurane in two CO2 absorbents during low-flow anesthesia in surgical patients. Anesthesiology. 1992; 77:1064–1069.
- J. L. Martin, L. Kandel, M. J. Laster, R. L. Kerschmann, E. I. Eger II.Studies of mechanism of nephrotoxicity of Compound A in rats. Journal of Anesthesia. 1997; 11:32-37.
- Bito H, Ikeda K. Closed-circuit anesthesia with sevoflurane in humans. Effects on renal and hepatic function and concentrations of breakdown products with soda lime in the circuit. Anesthesiology. 1994; 80:71–76.
- Kharasch ED, Frink EJ Jr, Zager R, et al. Assessment of low-flow sevoflurane and isoflurane effects on renal function using sensitive markers of tubular toxicity. Anesthesiology. 1997; 86:1238–1253.
- Litz RJ, Hübler M, Lorenz W, et al. Renal responses to desflurane and isoflurane in patients with renal insufficiency. Anesthesiology. 2002; 97:1133–1136.
- Ebert TJ, Frink EJ Jr, Kharasch ED. Absence of biochemical evidence for renal and hepatic dysfunction after 8 hours of 1.25 minimum alveolar concentration sevoflurane anesthesia in volunteers. Anesthesiology. 1998; 88:601–610.
- Holak EJ, Mei DA, Dunning MB 3rd, et al. Carbon monoxide production from sevoflurane breakdown: Modeling of exposures under clinical conditions. Anesth Analg. 2003; 96:757–764.
- Fang ZX, Eger EI 2nd, Laster MJ, et al. Carbon monoxide production from degradation of desflurane, enflurane, isoflurane, halothane, and sevoflurane by soda lime and Baralyme. Anesth Analg. 1995; 80:1187–1193.
- Pharmacology Mentor. Available from: https://pharmacologymentor.com/inhalation-anesthetics. Accessed May 1, 2025.
- Sulbaek Andersen MP, Nielsen OJ, Sherman JD. Assessing the potential climate impact of anaesthetic gases. Lancet Planetary Health. 2023.
- Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. Jul 2010; 111(1):92-98.
- NOAA research. 2024. Available from: https://research.noaa.gov/nitrous-oxide-emissions-grew-40-percent-from-1980-to-2020-accelerating-climate-change/. Accessed May 1, 2025.
- ASA Inhaled Anesthesia Climate Challenge:https://www.asahq.org/about-asa/governance-and-committees/asa-committees/environmental-sustainability/inhaled-anesthetic-challenge. Date of last update: April 21, 2025.