UDK: 616.12-008.313-073.432.19-032:611.329
https://www.doi.org/10.55302/MJA2484142z
Zilberman P1, Benhamou D2, Van Zundert A3
1Department of Anesthesia, Hadassah Medical Centre, Mount Scopus Campus, Jerusalem, Israel.
2Dan Benhamou. Service d’Anesthésie Réanimation Médecine Péri Opératoire. Université Paris Saclay. Hôpital Bicêtre – 78, rue du Général Leclerc. 94275 Le Kremlin Bicêtre Cedex, France.
3Department of Anesthesia and Perioperative Services, Royal Brisbane and Women’s Hospital & The University of Queensland, Brisbane, Queensland, Australia.
Case Presentation
A 76-years-old woman, 165cm, 53kg, ASA 2, was scheduled for a Watchman procedure, i.e. closure of the left atrial appendage (LAA) in patients with chronic atrial fibrillation, particularly in patients who cannot be treated with anticoagulants (1). The procedure was performed by advancing and deploying a device with a spring or umbrella-like structure into the right atrium under transesophageal echocardiography (TEE) guidance through the interatrial septum (1).
The patient recently experienced a hemorrhagic transient ischemic attack, while on apixaban. Although she recovered without complications, anticoagulation was discontinued due to risks causing another hemorrhagic event with potentially unpredictable outcomes.
As an alternative to anticoagulation, the patient agreed to a Watchman procedure.
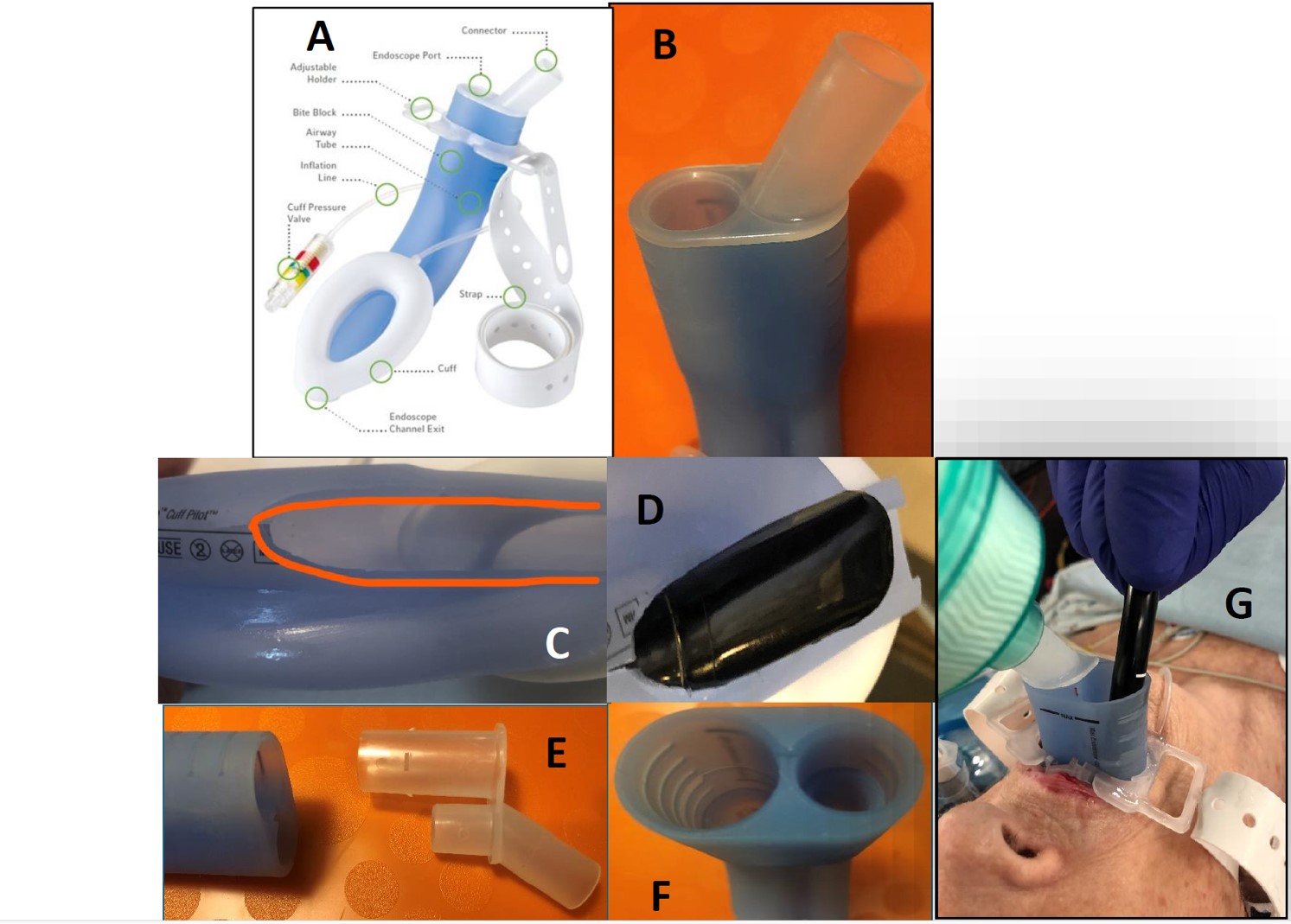
The interventional scenario was complicated by the patient’s history of pneumonectomy for lung cancer, which had resulted in paralysis of the ipsilateral vocal cord. The patient refused endotracheal intubation, fearing damage to her remaining functional vocal cord. As an alternative, the LMA-Gastro® (Teleflex®, Athlone, Ireland) (Figure 1A+B) was selected, allowing for both airway management (ventilation/ respiration) and insertion of the TEE probe (2). All LMA-Gastro sizes can accommodate endoscopes that fit in a 14mm diameter channel and a 2D-TEE trial (GE6VT-D, 14mm) passed through the endoscopic port (Figure 1C+D), but the LMA shape created friction that impeded free manipulation. As a result, the first modification was made as previously described (3). On the procedure day, the use of a 3D-TEE probe (17mm transverse dimension) was necessary, but it could not pass through the endoscopic port, and an additional modification was necessary. The breathing port and endoscopic port were built together, but the latter extends by 4cm into the body of the LMA to create a bite bock. The two ports were separated, leaving only the breathing port (Figure 1C) connected to the body of the LMA-Gastro®, and allowing the 3D-TEE probe to easily pass through the now-empty gastric entry channel (Figure 1E-1F-1G).
After Intravenous induction of GA modified LMA-Gastro® (size 3) was inserted, ventilation was assessed. A reliable ETCO2 trace was observed on the monitor and mechanical ventilation was initiated with volume-controlled ventilation and a positive end-expiratory pressure of 5cm H2O under oxygen and sevoflurane. The procedure went uneventful.
Figure 1. LMA-Gastro® Teleflex
- Picture from the official Teleflex website showing the commercially available LMA-Gastro®. Image courtesy of Teleflex Incorporated 2024. All rights reserved.
- Picture centered on the proximal part of the mask, showing the two-port connector (ventilation connector and endoscope port).
- Top view of device, showing the modified LMA-Gastro® with manual removal of the distal part and back part allowing to accommodate up to a 14mm tube.
- Endoscopic 2D TEE probe passing through the 14mm endoscope channel exit in the dry test.
- Proximal part of the LMA-Gastro® with the proximal two-port connector removed.
- Proximal part of the LMA-Gastro® after the removal of the connector. The large diameter channel is the endoscopic one.
- The LMA Gastro® in a ventilated patient after withdrawing the endoscope connector
Discussion
The procedure was elective but with a sense of urgency because no anticoagulants were given. At the time this patient was cared for, no valuable alternative method was available. The new airway device (Jcerity Endoscoper Airway® – JEA, Zhejiang Jcerity Medical Technology, Huzhou, China) is now available and its 20mm endoscopic opening would have easily accommodated the 3D-TEE probe (4). It also has an open built-in back opening which is similar to modification previously made manually (3). This device was not available to us and may still be restricted to use only in China. Although the Gastro-Laryngeal Tube® was also available and presents with a port dedicated for inserting a gastro-duodenal endoscope, the internal diameter of its endoscopic port is 13.8mm and would not have allowed to pass the large 3D-TEE tube. The modified LMA-Gastro® was our only option.
Aminian et al. recently described applying smaller 2D TEE probes (external diameter 8mm), allowing LAA closure to be performed under sedation. However, only the standard large TEE probe allows 3D imaging. If the 3D-TEE is required, GA may be necessary and the diameter of the endoscopic port of the airway device should be large enough to accommodate such a probe (5).
Managing this case required quick adaptation of an existing device to meet an unforeseen challenge. This situation demonstrates how flexible, “out-of-the-box” thinking can lead to successful outcomes and potentially inspire new applications for the LMA-Gastro®.
Acknowledgements
We acknowledge the dedicated colleagues from The Hadassah Medical Centre, both Campuses, whose expertise ensured the success of this procedure: Dr. Boaz Kalush (Anesthesia), Prof. David Leibovitz (Cardiology), Dr. Elhanan Fried (ICU), Prof. Ronen Durst (Cardiology), Dr. Yitzhak Biton (Cardiology).
We thank Teleflex for allowing the use of picture of the LMA-Gastro® (Figure 1A).
Image courtesy of Teleflex Incorporated 2024. Teleflex Incorporated. All rights reserved.
The patient gave consent to the publication of her clinical history.
Key Words: endoscopy; laryngeal mask airway; left atrial appendage closure; transesophageal echocardiography.
References:
- Khan S, Naz H, Khan MSQ, Ullah A, Satti DI, Malik J, Mehmoodi A. The WATCHMAN Device Review: A New Era for Stroke Prophylaxis. J Community Hosp Intern Med Perspect 2023;13(3):10-20. https://doi: 10.55729/2000-9666.1183. eCollection 2023.
- Hagan KB, Coronel E, Ge P, Hagberg C. A randomized controlled trial of the LMA® Gastro™ compared to nasal cannula for endoscopic retrograde cholangiopancreatography. Anaesth Crit Care Pain Med 2024;43(4):101379. https://doi.10.1016/j.accpm.2024.101379.
- Zilberman P, Davidovics Z, Benson AA. A bench test of a modified gastro LMA for the insertion of the duodenoscope. Indian J Anaesth 2022;66(2):159-60. https://doi.10.4103/ija.IJA_179_21.
- ZhouJ, LiL, Wang F, Lv Y. Comparison of the Jcerity Endoscoper Airway with the LMA supreme for airway management in patients undergoing cerebral aneurysm embolization: a randomized controlled non-inferiority trial. BMC Anesthesiol 2022;22(1):121. https://doi.10.1186/s12871-022-01666-w” https://doi.10.1186/s12871-022-01666-W.
- Aminian A, Leduc N, Freixa X, Swaans MJ, et al. Left Atrial Appendage Occlusion Under Miniaturized Transesophageal Echocardiographic Guidance and Conscious Sedation: Multicenter European Experience. JACC Cardiovasc Interv 2023;16(15):1889-98. https://doi.10.1016/j.jcin.2023.06.007.