Popovska H1, Boshkovski T2, Petrov I1, Popevski – Dimovski R3
1University “Ss. Cyril and Methodius”, Medical Faculty, Skopje, Macedonia
2Brainster Next College, Skopje, Macedonia
3 University “Ss. Cyril and Methodius”, Faculty of Natural Sciences and Mathematics, Skopje, Macedonia
UDK: 616.858:159.922.072
https://www.doi.org/10.55302/MJA2481047p
Abstract
Introduction: Cognitive deficit is a common non-motor manifestation in patients with Parkinson’s disease (PD). Over time, about 80% of PD patients develop dementia. The presence of a mild cognitive deficit, which does not interfere with daily activities, represents a high risk for conversion to dementia. There is a growing interest in the potential of neuroimaging techniques to develop non-invasive biomarkers in neurodegenerative disease, specifically in Parkinson’s disease.
Objective: The main objective in this study is to find imaging biomarker that will differentiate patients with Parkinson’s disease based on their cognitive status.
Methods: Thirty subjects participated in the current study and were grouped into 3 groups. The first group consisted of 10 healthy individuals, used as a control group. In the second group, 10 patients with Parkinson’s disease and normal cognitive status were involved. Finally, the third group consisted of 10 patients with Parkinson’s disease and mild cognitive deficit. The cognitive status of all subjects was evaluated using neuropsychological tests. Additionally, for all subject rs-fMRI was acquired to reconstruct the functional connectome. The edges from the reconstructed functional connectome were used as classification features to discriminate between the different groups.
Results: It was found that the global node degree of functional connectome is associated with decreased performance in some domains of cognitive function, like memory and executive functions.
Conclusion: The patterns of functional connectivity may be useful in discrimination patients with Parkinson’s disease based on the presence of cognitive deficits.
Key Words: Functional connectomes, mild cognitive impairment, rs-fMRI, Parkinson’s disease.
Introduction
Parkinson’s disease (PD) is considered as an expression of diffuse neurodegeneration, which affects the peripheral and central nervous system. PD is a progressive alpha-synucleinopathy, which manifests characteristically with a wide range of appendicular, axial motor symptoms, accompanied in some cases by non-motor symptoms (1,2).
When talking of cognition in PD, the most frequently affected domains are executive function, attention, memory. The term “mild cognitive impairment” (MCI), in a patient with PD, refers to clinically evident cognitive impairment without functional decline (3,4), and therefore associated with a higher risk of developing dementia (5).
Pathologically in PD alpha-synucleinopathy with Lewy pathology develops in predictive stages. According to the leading hypothesis the process starts from the olfactory bulbs and dorsal motor nucleus of the vagus nerve (stage 1), then spreads to the rest of the brainstem nuclei and basal ganglia (stages 2 – 4) and finally to the neocortex (stages 5 and 6). The first signs of motor parkinsonism can be expected in stage 4 and/ or 5, when the loss of nigral dopaminergic cells exceeds the clinical threshold. With involvement of the neocortex, cognitive changes are expected (6,7).
However, during the new era, revision and update of diagnostic criteria for Parkinson’s disease which include the cause of parkinsonism are published (8). This also emphasized that a clinical diagnosis of MCI in patients with Parkinson’s disease, neuropsychological tests, which cover every domain of cognition, should be used. The most commonly neuropsychological tests that should be used are: Episodic memory – Rey-auditory test for remembering 15 words, with immediate recall and with delayed recall (9); Execution – Frontal Assessment Battery (FAB) (10) Stroop-color-word test (11); Attention- matrices for attention (12); Visuo-spatial domain- redrawing of the Rey-Osterrieth complex figure (13), the clock test (14) and for global cognition – Mini Mental State Examination (MMSE) (15).
On the other hand, finding an imaging biomarker that will differentiate patients with Parkinson’s disease based on their cognitive status is still controversial. The functional magnetic resonance imaging (fMRI) is a technique that offers a non-invasive access to brain function, based on the changes of the blood-oxygen level dependent (BOLD) signals, that are indirectly associated with functional brain activity. In the human brain specific functions are localized to different parts of the brain and can be identified by fMRI and mapped at higher spatial resolution (16,17). Two fMRI techniques are used to study the brain function: resting state fMRI (rs-fMRI), and task-based fMRI, both determine different BOLD changes.
In recent years, graph theory has been used to understand the global topological organization of brain networks by applying this approach to rs-fMRI imaging. This study has shown the existence and properties of the default mode network (DMN). It is a neural network of apparent resting brain states. Because DMN was first identified with the resting state, it has been appealing to many to associate DMN’s function with the mental state that commonly accompanies a relaxed state, namely daydreaming, mind wandering or stimulus-independent thoughts (19). Furthermore, functional dysconnectivity detected before the occurrence of neuronal death and brain atrophy, has a potential to serve as a sensitive marker of pathological processes (20-22).
Therefore, for the patients with PD it is important to identify characteristic patterns of functional connectivity between specific brain regions with and without mild cognitive decline. So, the objective of this study is to find imaging biomarker (MRI)) that will differentiate patients with Parkinson’s disease based on their cognitive status.
Methods and Material
This is a pilot study that was done after approval of the ethics committee of the Faculty of Medicine, University “Ss. Cyril and Methodius”, Skopje. The pilot study included 30 patients divided into three groups. Group (PD-nonMCI)* included 10 patients with Parkinson’s disease without mild cognitive impairment, group (PD-MCI)* included 10 patients with Parkinson’s disease with mild cognitive impairment and control group consisted of 10 healthy individuals. All patients were recruited from the Neurology Clinic in Skopje, while the healthy individuals volunteered to participate in the study. All subjects have signed an informed consent to participate. To be included in the PD groups, patients had to had occurrence of Parkinson’s disease starting after the age of 50, stage 1 and 2 of the disease according to the scale of Hoehn and Yahr (23), antiparkinsonian treatment** (24) started at least 4 weeks before entering the study. Patients with Parkinson’s disease with diagnosed dementia, psychiatric diseases, medicines that potentially interfere with cognition, including psychotropic substances and anticholinergic drugs, and patients with serious cardiovascular or cerebrovascular diseases were excluded from the study.
Study protocol: all study’s subjects underwent standardized study protocol (neuropsychological assessment and Magnetic Resonance Imaging MRI).
Neuropsychological assessment: The same psychological tests were administered to all subjects with Parkinson’s disease. The neuropsychological tests were grouped according to cognitive function as follows: 1. Episodic memory – Rey auditory test for remembering 15 words, with immediate recall and with delayed recall; 2. Execution – Frontal Assessment Battery (FAB), Stroop-color-word test; 3. Attention-matrices for attention and 4. Visuo-spatial domain redrawing of the Rey–Osterrieth complex figure, the clock test. All participants were assessed with the Mini Mental State Examination (MMSE).
MRI protocol: All participants were scanned with a 3T SIEMENS Prisma Scanner, using the following multimodal protocol: 1. DTI sequence (TR=12.5ms, TE=89ms, voxel size=2x2x2mm3, gradient direction=30, maximum b value=1000s/mm2), 2. MPRAGE sequence (TR=2200ms, TE=2.26ms, flip angle= 8 degrees, TI=950ms, FOV=256x256mm, voxel size=1x1x1mm3), 3. Rs-fMRI sequence (TR=2550ms, TE=25ms,flip angles=90 degrees, time points=220, voxel size=2.8×2.8×2.8mm3).
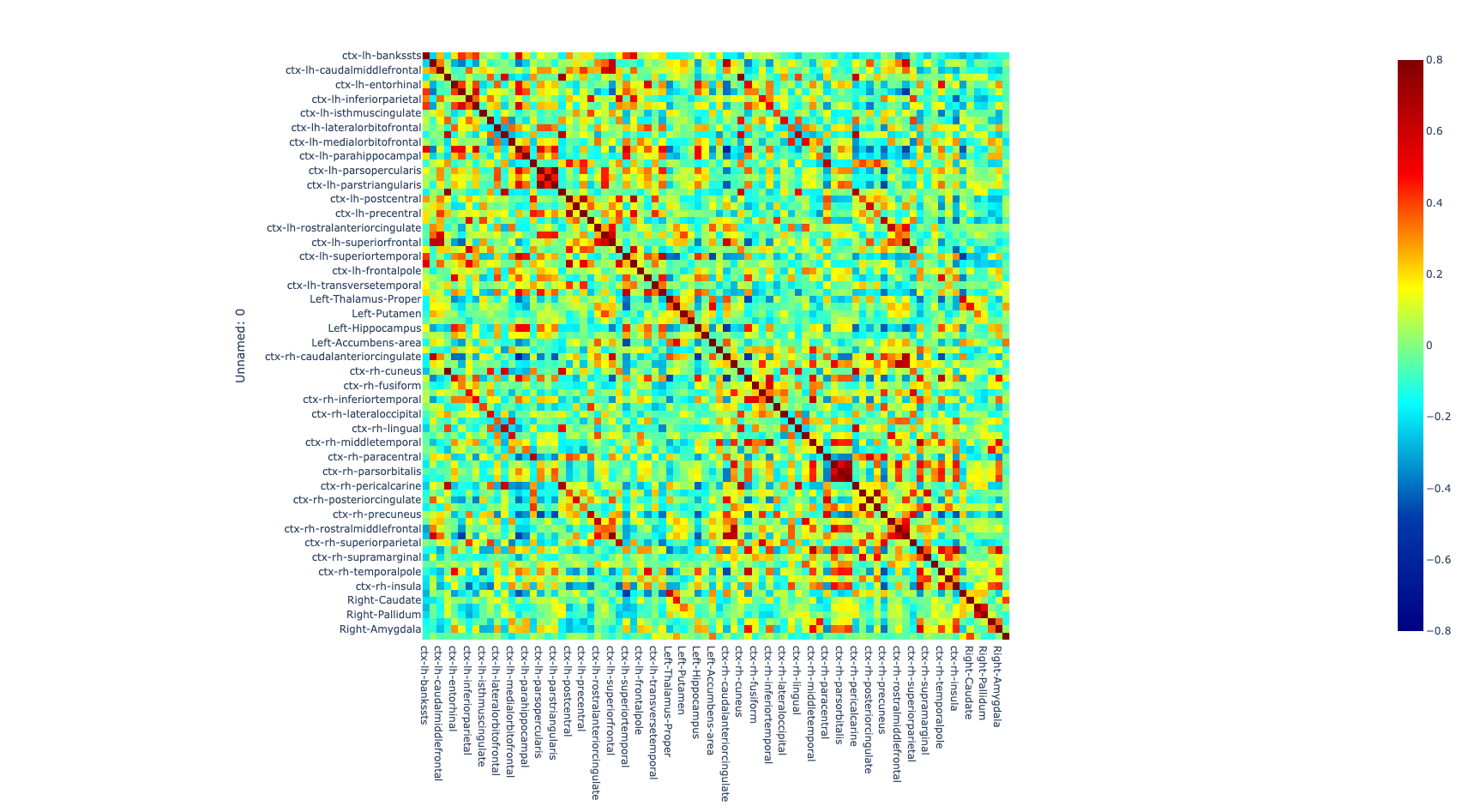
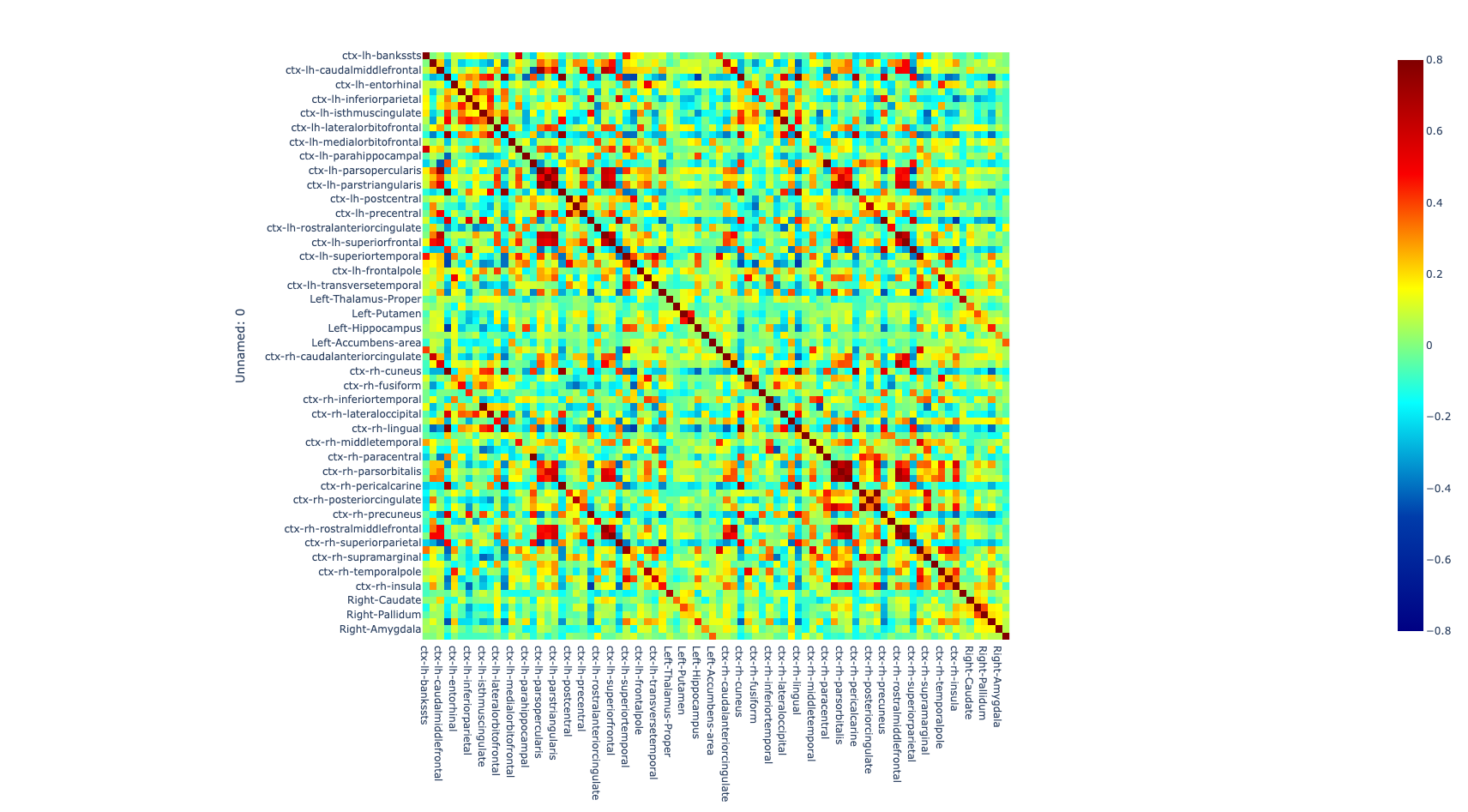
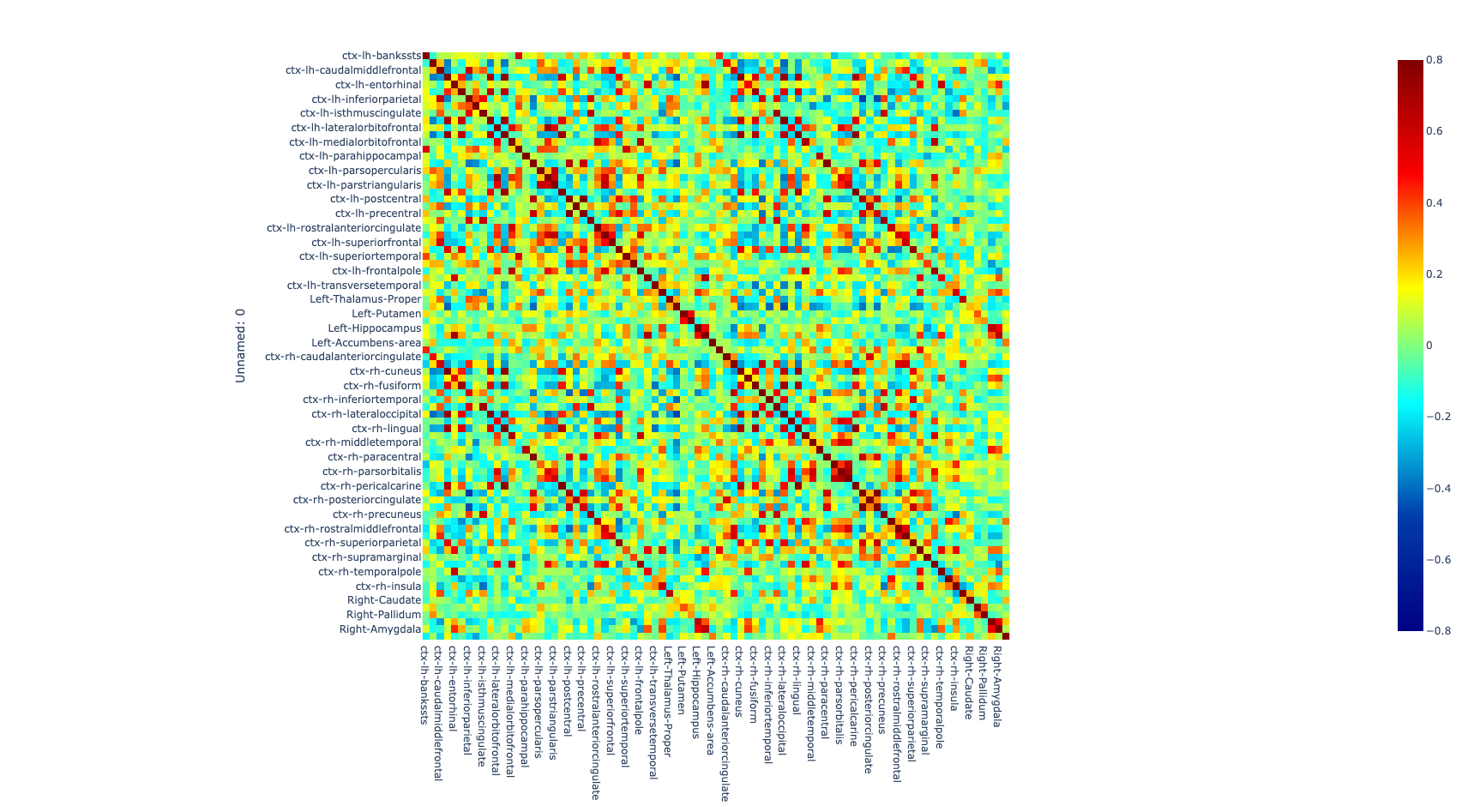
Once the images for all subjects were acquired, a quality check was performed by a trained neuroscientist, and only the images that have passed the QC were analyzed. After the QC, the images were preprocessed. The T1w image was processed with freesurfer (ref) to parcellate the brain into 68 cortical and 14 subcortical cerebral gray matter regions using the Desikan-Killiany Atlas (26). Additionally, the rs-fMRI images were also preprocessed to correct for some of the most common artifacts slice timing, motion, and susceptibility-based artifact. The fmriprep (ref) pipeline was used to perform the preprocessing on the rs-fMRI images.
After correcting for the bias in the fMRI images caused by artifacts, both preprocessed T1w and rs-fMRI images were registered to standard MNI (Montreal Neurological Institute) space using a non-linear transformation as implemented in ANTs (27). Then, the average time series of all voxels included in each region were extracted and the partial correlation was computed between the time series of each pair of regions. The individual brain network was defined with 68 cortical and 14 subcortical brain regions as nodes and 3,362 unique interconnection links. In order to obtain characterization of connectome differences between groups, we looked at the topological organization of the brain network (28, 29).
* Patients were evaluated according to diagnostic criteria for PD (MDS-PD), as well as with neuropsychological assessment for their cognitive status, using multiple neuropsychological tests for different domains of cognition.
** For antiparkinsonian treatment was considered antiparkinsonian drugs, various combinations of levodopa, dopamine agonists, catechol-O-methyl transferase inhibitors, monoamine oxidase inhibitors, amantadine. Levodopa equivalent daily dose (LEDD) was estimated in a way suggested by Tomlison et al (24).
Results
Demographic and Neurocognitive Characteristic
The subjects were evaluated using the MDS-UPDRS. Also, the subjects were assessed for their cognitive abilities using the already named neuropsychological tests. By using the Kruskal-Wallis nonparametric test, we compared the demographic and the clinical scores (Table 1). With a χ2 test, the gender was compared.
In all three groups, there was no significant difference in demographic characteristics (p>0.05). However, there was observed a significant difference between the groups for the MMSE, the tests of episodic memory, executive function and visuospatial abilities, whereas the group with PD and with mild cognitive decline had significant lower scores (Table 1).
Table 1. Demographic and cognitive outcomes.
| Control group (n=10) | PD-nonMCI (n=10) | PD-MCI
(n=10) |
p-value | |
| Age | 63.3 (10.3) | 63.6 (9.5) | 66.5 (11.1) | 0.404 |
| Gender (female) | 7 | 9 | 9 | 0.796 |
| Hand dominance (right) | 10 | 10 | 10 | 0.495 |
| MDS-UPDRS | – | 14.6 (7.2) | 16.8 (11.0) | 0.358 |
| LEDD | – | 5.9 (4.0) | 10.1 (7.2) | 0.013 |
| MMSE | 29.7 (0.5) | 29.4 (0.7) | 28.6 (1.5) | 0.001 |
| Episode memory | -0.01 (0.56) | -0.06 (1.04) | -0.91 (0.86) | <0.001 |
| Execitive function | 0.04 (0.64) | -0.02 (0.65) | -1.69 (1.80) | <0.001 |
| Attention | 0.06 (0.75) | 0.13 (0.68) | 0.31 (0.75) | 0.366 |
| Visuospatial score | -0.02 (0.8) | -0.23 (0.62) | -1.53 (1.05) | <0.001 |
MDS-UPDRS, Movement Disorder Society Unified Parkinson’s Disease Rating Scale; LEDD, Levodopa equivalent daily dose; MMSE, Mini-Mental State Examination; PD-nonMCI, Parkinson’s disease-non-Mild Cognitive Impairment; PD-MCI, Parkinson’s disease-Mild Cognitive Impairment.
Imagining and fMRI Data
Figure 1. Global metrics and intersubject correlation among different scans at different connectivity densities. Functional connectivity networks at different connectivity densities. For each scan and each individual, three essential properties of the voxel-based functional networks were calculated at different connectivity densities, including the mean functional connectivity strength (FCS), the largest component size (LCS) and the number of isolated nodes.
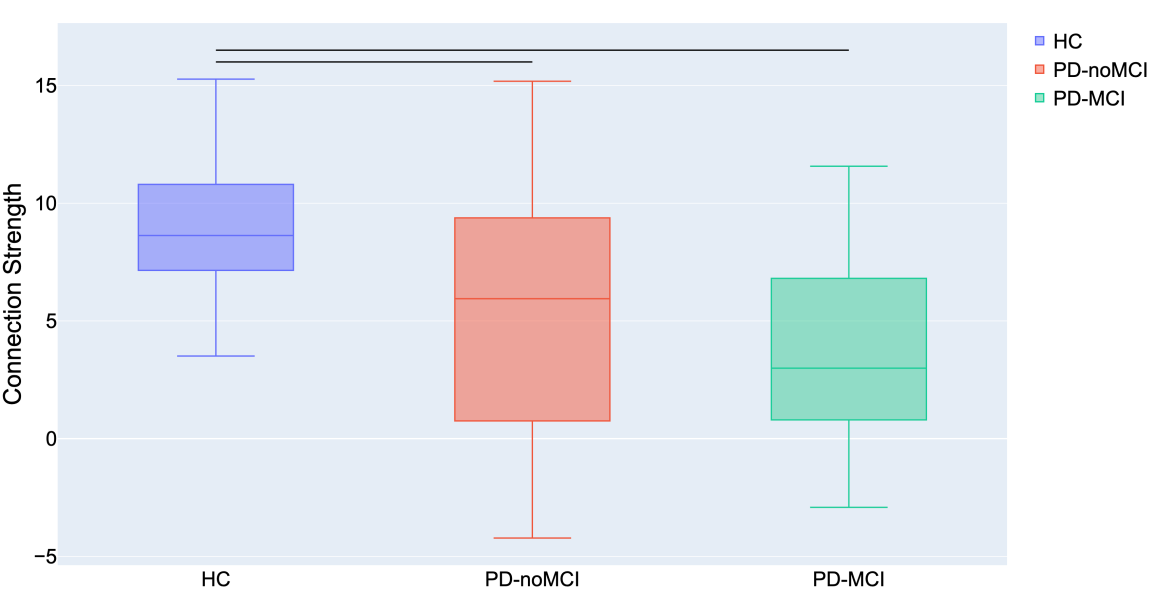
The comparison of connectomes between the groups was done by looking at the average nodal strength. We found a significant reduction of the average connection strength in patients with PD-MCI versus the control group (p<0.01) and in patients with PD without MCI versus the control group. No significant difference between PD without MCI with MCI (Pearson’s correlation coefficient).
|
Discussion
Using functional connectomes, we achieved high accuracy in classifying Parkinson’s disease patients with and without mild cognitive deficit. Even though our study included small number of patients, the results suggest that functional connectivity patterns can be used to determinate and differentiate Parkinson’s disease patients based on their cognitive status. In this study, significant changes in connectivity were observed in patients with Parkinson’s disease especially in the mild cognitive deficit (PD-MCI) group. This is consistent with findings from previous studies even though different numbers of patients are used in both studies (30,31).
With new neuroimaging biomarkers sensitive to cognitive impairment in neurodegenerative disease, scientists may have the ability to predict cognitive decline, to identify at-risk patients who could benefit from potential early treatment (32).
Neuroimaging techniques such as fMRI have traditionally been used to study the pathophysiology of brain disorders by comparing patient groups with healthy groups (33,34). A growing number of studies have attempted to develop prognostic/ diagnostic tool through neuroimaging techniques. To become clinically useful, findings from such studies need to demonstrate reproducibility and generalizability (35,36). However, many fMRI studies have failed to be replicated. The use of non-standardized sequences (37), the lack of standardization of the steps in image preprocessing (38), as well as flexibility in data collection, analysis and reporting of results (39), are some potential culprits for this problem in replication.
In our study, we assessed the generalizability of our findings using an independent validation sample. The validation dataset was obtained using identical fMRI acquisition parameters, image preprocessing protocol and analytical methods.
Study has limitations. This is a small sample of subject size, so the results even though were elaborated and corrected adequately from statistic expert, we must consider that Parkinson disease is not the most frequent disease and additionally in this study diagnostic tools are not completely standardized, and this is the first study from the Republic of North Macedonia that functionally encopresis so many variables. This study needs and opens a door for larger randomized study to determine the early recognition of patients with PD and functional brain deficit, to have better treatment outcome in these patients.
Conclusion
From the analysis, it was shown that multivariate resting-state functional connectivity models can be used to differentiate Parkinson’s disease patients according to their cognitive status, using a fMRI. The resulting data obtained from functional connectomes, have the potential of sensitive biomarkers for the extent of cognitive impairment in patients with Parkinson’s disease. Still larger studies are needed.
References:
- Muslimovic D, Post B, Speelman JD, Schmand B.Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 2005; 65:1239–1245.
- Barone P, Aarsland D, Burn D, Emre M, Kulisevsky J, Weintraub D. Cognitive impairment in nondemented Parkinson’s disease. Mov Disord 2011; 26:2483–2495.
- Caviness JN, Driver-Dunckley E, Connor DJ et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord 2007; 22:1272–1277.
- Litvan I, Goldman JG, Troster AI et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement disorder society task force guidelines. Mov Disord 2012; 27:349–356.
- Broeders M, de Bie RMA, Velseboer DC et al. Evolution of mild cognitive impairment in Parkinson disease. Neurology 2013; 81:1–7.
- Robert EB, William TD, Jean Paul GV. A Critical Evaluation of The Braak Staging Scheme for Parkinson’s Disease. Ann Neurol. 2008 Nov; 64(5): 485–491.
- Kouli A, Torsney KM, Kuan WL. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In: Stoker TB, Greenland JC, editors. Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]. Brisbane (AU): Codon Publications; 2018 Dec 21. Chapter 1. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536722.
- Ronald BP, Berg D et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591-601.
- Caltagirone C, Gainotti G, Masullo C, Miceli G. Validity of some neuropsychological tests in the assessment of mental deterioration. Acta Psychiatr Scand 1979; 60:50–56.
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology 2000; 55:1621–1626.
- Barbarotto R, Laiacona M, Frosio R, et al. A normative study on visual reaction times and two Stroop colour-word tests. Ital J Neurol Sci.1998; 19:161–170.
- Spinnler H, Tognoni G. Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci.1987; 6(8):1–20.
- Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A. Rey–Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci.2002; 22:443–447.
- Rusconi, M. L., Fusi, G., Stampatori, C., Suardi, A., Pinardi, C., Ambrosi, C., Costa, T., & Mattioli, F. Developmental topographical disorientation with concurrent face recognition deficit: Frontiers in Psychiatry,2021 12,Article 654071. https://doi.org/10.3389/fpsyt.2021.654071 .
- Davey RJ, Jamieson S. The validity of using the mini mental state examination in NICE dementia guidelines. J Neurol Neurosurg Psychiatry. 2004; 75:343-44.
- Smith, S. M. et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci.2009; 106:13040–5.
- Sporns, O., Tononi, G. & Kцtter, R. The human connectome: A structural description of the human brain. PLoS Comput. Biol.2005;1, e42.
- Marcus E.Raichle. The Brain’s Default Mode Network. Annu.Rev.Neurosci.2015;38:433-47.
- Baggio, H.-C. et al. Functional brain networks and cognitive deficits in Parkinson’s disease. Hum. Brain Mapp.2014; 35: 4620–34.
- Rosa de Micco et al. Functional Connectomics and Disease Progression in Drug-Naïve Parkinson’s Disease Patients. Mov.Dis. 2021.36, 1603-16.
- Olde Dubbelink, K. T. E. et al. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology .2014;83:2046–53.
- Amboni, M. et al. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson’s disease. J. Neurol. 2015; 262:425–34.
- Pablo Martinez‐Martin MD et al. Validation study of the Hoehn and Yahr scale included in the MDS‐UPDRS. Movement Disorders, 2018; 33(4): 134-165.
- Tomlinson, C. L. et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord.2010; 25: 2649–2653.
- Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52: 1059–69.
- Fan, L. et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb. Cortex 2016; 26: 3508–3526.
- Behzadi, Y., Restom, K., Liau, J. & Liu, T. T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007; 37, 90–101.
- Friston, K. J., Rotshtein, P., Geng, J. et al. A critique of functional localizers. Neuroimage 2006; 30: 1077–87.
- Zalesky, A, Fornito, A, Bullmore, ET. Network-based statistic: identifying differences in brain networks. Neuroimage 2010; 53: 1197–207.
- Amboni, M. et al. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson’s disease. J. Neurol.2015; 262:425–34.
- Gorges, M. et al. To rise and to fall: functional connectivity in cognitively normal and cognitively impaired patients with Parkinson’s disease. Neurobiol. Aging 2015; 36: 1727–1735.
- Dickerson, B. C. & Sperling, R. A. Neuroimaging biomarkers for clinical trials of disease-modifying therapies in Alzheimer’s disease. NeuroRx 2005; 2:348–60.
- Garcia-Garcia, D. et al. Posterior parietooccipital hypometabolism may differentiate mild cognitive impairment from dementia in Parkinson’s disease. Eur. J. Nucl. Med. Mol. Imaging 2012; 39:1767–77.
- Dickerson, B. C. & Sperling, R. A. Neuroimaging biomarkers for clinical trials of disease-modifying therapies in Alzheimer’s disease. NeuroRx 2005;2: 348–60.
- Arbabshirani, M. R., Plis, S., Sui, J. et al. Single subject prediction of brain disorders in neuroimaging: Promises and pitfalls. Neuroimage 2017; 145:137–165 (2017).
- Calhoun, V. D. & Lawrie, S. M. Prediction of Individual Differences from Neuroimaging Data. NeuroImage 2017; 145: 135–136.
- 37.Teipel, S. J. et al. Multicenter stability of resting state fMRI in the detection of Alzheimer’s disease and amnestic MCI. NeuroImage Clin, 2017 Jan 18;14:183-194.doi: 10.1016/j.nicl.2017.01.018.
- Vergara, V. M., Mayer, A. R., Damaraju, E., Hutchison, K. & Calhoun, V. D. The effect of preprocessing pipelines in subject classification and detection of abnormal resting state functional network connectivity using group ICA. Neuroimage 2017;145: 365–376.
- Poldrack, R. A. et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci,2017;18, 115–126. doi: 10.1038/nrn.2016.167.