UDK: 502.175:[614.72:616-089
Lleshi A.1, Brzanov Gavrilovska A.1, Kiprijanovska B.1, Ognjanova V.1, Trposka A.1, Mladenovska Cvetkova M.1
1 University Clinic for Traumatology, Orthopedic Diseases, Anesthesiology, Reanimation and Intensive Care Medicine and Emergency department, Clinical Center Mother Theresa Faculty of Medicine, “Ss. Cyril and Methodius” University Skopje, Republic of North Macedonia
Abstract
Introduction: The stress response to surgery, a sequence of pathological and physiological alterations brought on by the stimulation of surgery, can be divided into two main categories: the inflammatory-immune response and the neuroendocrine-metabolic response. It depends on the anesthesia technique and surgical approach.
Material and Methods: The patients were divided into two groups: Sevoflurane inhalational anesthesia (SIA) and Target control infusion-Total intravenous anesthesia (TCI-TIVA). The TCI-TIVA group has used the Marsh model for the propofol and the Minto model for the remifentanil, using target plasma concentration. The SIA group has induction in general anesthesia with Propofol plus Fentanyl, and the maintenance of anesthesia has been achieved with Sevoflurane on MAC 0.7-1.0. We compared the effect of different anesthetic techniques on the surgical stress response through measuring the blood levels of proinflammatory cytokines Interleukin-6, Cortisol and blood glucose, as well as the hemodynamic response.
Results: Interleukin-6 (IL-6) levels rise sharply from T0 (4.78εg/mL) to T2 (10.06εg/mL) and then again at T3 (36.34εg/mL), showing a strong inflammatory response after surgery in the SIA group. IL-6 levels in the TCI-TIVA Group exhibit a comparable pattern, however with a significantly smaller increase at T3 (14.56). When comparing the cortisol levels at T0, both groups show a comparable range of variability. There is a highly significant difference in cortisol levels between TIVA and SIA after extubating and 24 hours postoperatively, as indicated by the T2 p-value of less than 0.001. Glucose levels in the SIA group are comparatively constant from T0 (5.29) to T1 (5.25), then they significantly rise at T2 (6.56) and stay high at T3 (5.71). Glucose levels in the TCI-TIVA Group exhibit less variability, increasing slightly from T0 (5.17) to T2 (5.21) and then staying constant at T3 (5.28). Hemodynamic stability was better with TCI-TIVA than with SIA.
Conclusion: Our findings indicate that TCI-TIVA consistently demonstrates advantages regarding controlling the stress response, inflammation, and metabolic response both during and after surgery as compared to the SIA group. These results provide credence to the prospective advantages of TCI-TIVA over SIA in surgical settings where patient’s outcomes depend critically on reducing stress, inflammation, and metabolic disturbances
Key Words: Blood glucose; Cortisol, Interleukin-6, Sevoflurane inhalational anesthesia, Stress response; Total intravenous anesthesia-target controlled infusion.
Introduction
Three phases characterize the host’s response to surgical trauma: the hypodynamic “ebb phase”, which appears in the early hours after tissue trauma, is followed by the hyperdynamic “flow phase”, which is hypercatabolic, and the third phase, which is thought to be the recovery period (2), during which the body attempts to restore homeostasis. Initially, corticotrophin-releasing hormone (CRH) is released in response to surgical stress, activating the hypothalamic-pituitary-adrenal (HPA) axis and resulting in metabolic alterations. Adrenocorticotropic hormone (ACTH) is secreted by the anterior pituitary gland in response to CRH. Cortisol release is stimulated by ACTH’s action on the adrenal cortex (1, 3). Blood glucose levels are raised when cortisol increases the liver’s gluconeogenesis. Depending on the severity of the surgical damage, the cortisol concentration rises a few minutes after surgery and peaks 4-6 hours later (1, 3, 4, 5). The particular immune response includes the generation of antibodies and cytokines. The main cytokines released after surgery are interleukin-1 (IL-1), tumor necrosis factor-a (TNF-a), and interleukin-6 (IL-6). IL-6 is the main cytokine that induces the acute-phase response or systemic changes. The first day after surgery is when cytokine levels are at their peak (1, 4).
The most common reason for breast surgery is to eliminate breast cancer, while benign tumor removal, abscess drainage, and cosmetic procedures are all common justifications. Breast reconstruction can take place either right after the original surgery or later after the malignancy is removed (6). The severity and length of operative trauma, surgical approach, and anesthetic type are some of the elements that influence the stress response to surgery (7). Options for anesthesia include a combination of intravenous (IV) and inhaled medications, or target control infusion-total intravenous anesthesia (TCI-TIVA) (6). The most effective kind of general anesthesia (GA) for patients having medical operations, is target control infusion-total intravenous anesthesia (TCI-TIVA) combined with remifentanil and propofol (RP). Commercially available pumps that employ target-controlled infusion (TCI) techniques to better regulate plasma concentration levels have led to increased safety and predictable timing (8). A TCI pump has an inbuilt microprocessor that is programmed with pharmacokinetic models for the right drugs. The TCI pump determines both the first bolus and continuous administration rates. The anesthesiologist chooses the medication and pharmacokinetic model based on the patient’s gender, age and body weight, as well as the target plasma concentration or target effect-site concentration “brain” (9). Target-controlled infusion (TCI) protocols prefer Remifentanil to other opioids for anesthesia because it has special qualities like starting to work quickly, being easy to control during surgery, and taking less time to recover (8, 10, 11). On the contrary, sevoflurane is a potent inhalation anesthetic due to its low blood/ gas solubility that allows for precise control of the anesthesia stage, quick and painless induction, and rapid recovery from anesthesia (12). The gold standard for figuring its potency is the minimum alveolar concentration (MAC) (13).
Using proper neuromonitoring is essential to determine the depth of anesthetic, confirm that the patient is asleep, and rule out pain in addition to non-invasive blood pressure and heart rate monitoring. In order to improve medication administration and anesthetic level, the electrical activity of people’s brains during sedation was measured to establish the Bispectral Index Scale (BIS) (14). The BIS provides anesthesia practitioners with valuable real-time input and represents a significant advancement in the objective evaluation of the level of anesthesia by reducing the chance of intraoperative awareness. This constitutes a substantial development in the objective evaluation of the level of anesthesia. A range of 40–60 BIS is suitable for general anesthesia, whether administered by TCI-TIVA or inhalational anesthesia (15).
This study’s main objective was to identify how the stress response to surgery was different for two types of anesthesia: Sevoflurane Inhalational Anesthesia (SIA) and Target-Controlled Infusion Total Intravenous Anesthesia (TCI-TIVA).
The study’s secondary objective was to investigate and compare the levels of three specific physiological markers: cortisol, Interleukin-6 (IL-6) and blood glucose.
Materials and Methods
Participants:
The patients in this prospective, randomized, interventional clinical study, were undergoing breast reconstructive surgery following a mastectomy for breast cancer. The study was conducted at the University Clinics for Plastic and Reconstructive Surgery, Skopje, and the University Clinic for Traumatology, Orthopedic Disease, Anesthesiology, Reanimation, Intensive Care Medicine and Emergency Department. The Bioethics Committee of the Medical Faculty in Skopje granted us approval. Written informed consent was signed by each patient before enrolling in the study. In this pilot trial, fifty patients were divided into two groups at random and given distinct general anesthetic protocols. The inclusion criteria were patients between the ages of 25 and 75, an ASA I–II classification and a BMI of less than 35 kg/m². Patients with a prior medical history of liver disease or preexisting liver dysfunction, renal insufficiency, diabetes mellitus, use of sedatives and opioids, or who refused to sign written informed consent were excluded from the study.
Procedures:
All operations began around 8:00 a.m. to avoid the variations in stress hormone levels. In order to maintain uniformity, every patient underwent anesthesia from the same anesthesia team for each procedure. Both groups had neuromonitoring with BIS, and when the BIS reading was below 60, muscle relaxant Rocuronium bromide at 0.6mg/kg was administered. After 90 seconds, using C-MAC video laryngoscopy, patients were intubated and mechanically ventilated on PC/VG mode with 2L flow, air and oxygen mixture, TV 6-8ml/kg, to maintain an end-tidal CO₂ range of 35–45mmHg. The respiratory rate was set between 10 and 14 breaths/min. BIS values were maintained between 40 and 60. During the procedure, each patient’s non-invasive blood pressure, heart frequency, and mean arterial pressure were monitored. Extubating occurred once the surgery was complete, the patient responded to verbal directions, and their tracheal and laryngeal reflexes recovered. Each patient received 50mcg of fentanyl after being extubated.
Intervention Conditions:
Patients were randomly divided into two groups, each receiving a distinct general anesthetic protocol:
Group TCI-TIVA: Patients in this group received general anesthesia with Target-Controlled Infusion-Total Intravenous Anesthesia (TCI-TIVA). The Marsh model was used for Propofol, and the Minto model for Remifentanil, using target plasma concentration.
Group SIA: Patients in this group received general anesthesia maintained with Sevoflurane Inhalational Anesthesia (SIA). Induction was with Propofol plus Fentanyl, and maintenance of anesthesia was achieved with Sevoflurane at MAC 0.7-1.0.
Measures:
At four distinct time intervals, blood samples were taken for laboratory testing in both groups:
T0: Before surgery; T1: After anesthesia induction in the operating room; T2: After extubating in the recovery room (PACU); T3: 24 hours after the conclusion of surgery.
Blood glucose levels, proinflammatory cytokines (cortisol and Interleukin-6), and blood flow response were monitored to evaluate the effects of the different anesthesia types on the postoperative stress response.
Statistical analysis:
Statistical analysis between two groups were performed with Student t-test. SPSS statistical software (version 27.0 SPSS, Inc., North Castle, NY) was used for the analysis; two-tailed P < 0.05 was considered significant. Data are shown as mean ± standard deviation if not otherwise stated.
Results
There were 40 participants in the sample. Demographic information was comparable for both groups. Each group’s average age was comparable 46±6.2 TCI-TIVA vs. 45±8.7 SIA group. Every patient in both cohorts was a female. The two groups’ body mass index (BMI) were similar 23±2.1 TCI-TIVA vs. 23±1,9 SIA group. Additionally, ASA classifications were reliable.
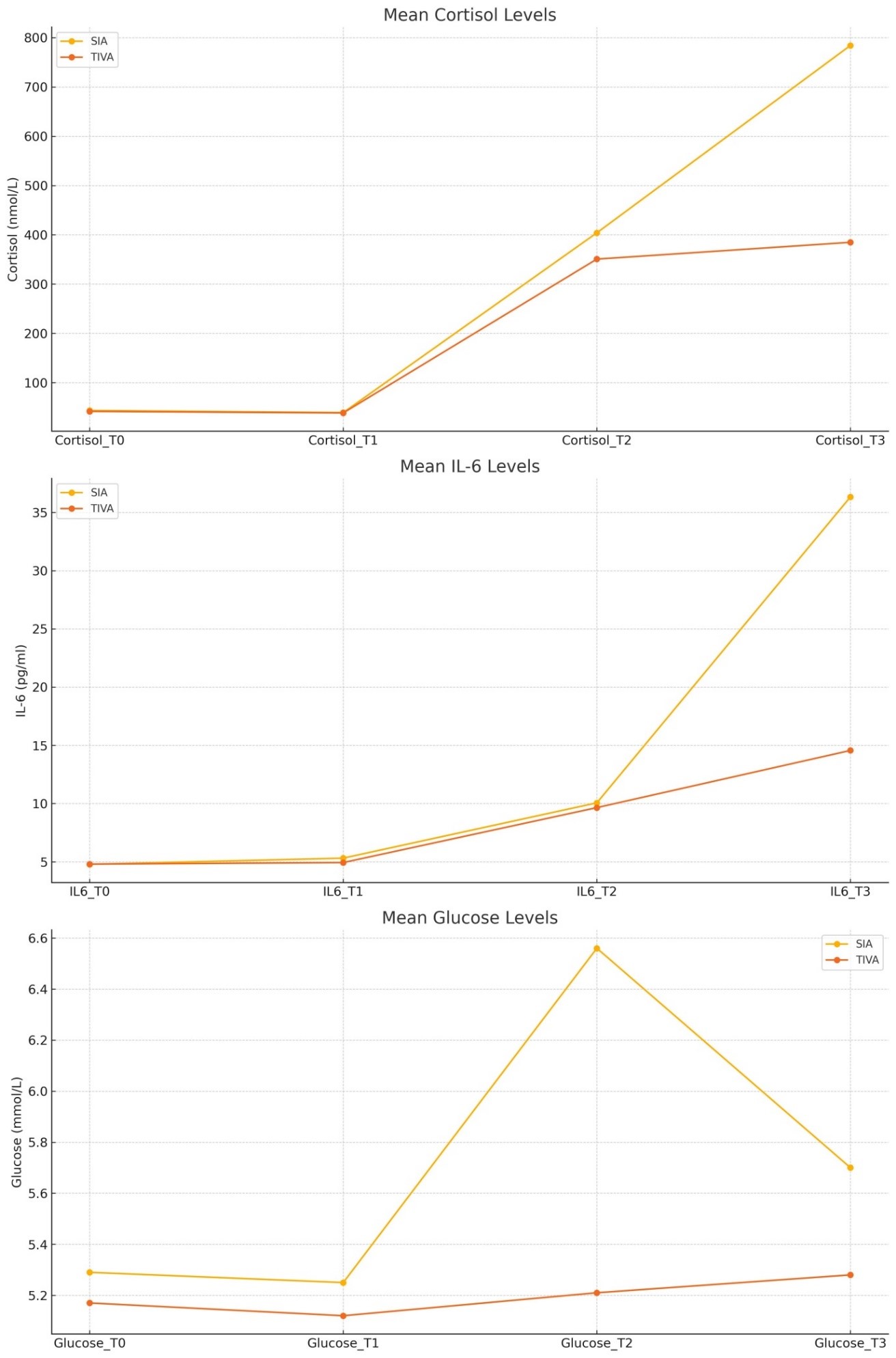
Interleukin-6 (IL-6) levels increase notably from T0 (4.78) to T2 (10.06) and then sharply at T3 (36.34), indicating a significant inflammatory response post-surgery in the SIA group. In the TCI-TIVA Group: IL-6 levels show a similar pattern but with a much lower increase at T3 (14.56) (Table 1).
Table 1. Comparison of IL-6 between SIA and TCI-TIVA.
| Type of Anesthesia | IL6_T0 | IL6_T1 | IL6_T2 | IL6_T3 |
| SIA | 4.78 | 5.31 | 10.06 | 36.34 |
| TCI-TIVA | 4.8 | 4.93 | 9.65 | 14.56 |
SIA-Sevoflurane inhalational anesthesia; TCI-TIVA-Target control infusion-Total intravenous anesthesia; IL-6 in pg/ml.
No significant difference in IL-6 levels is observed in TO, T1 and T2 between the two groups (p > 0.05), while a significant difference is observed in IL-6 levels 24 hours post-surgery (p < 0.001), with the TCI-TIVA group showing lower levels compared to the SIA group (Figure 1).
Figure 1. IL-6 (pg/ml) levels in T0, T1, T2 and T3.
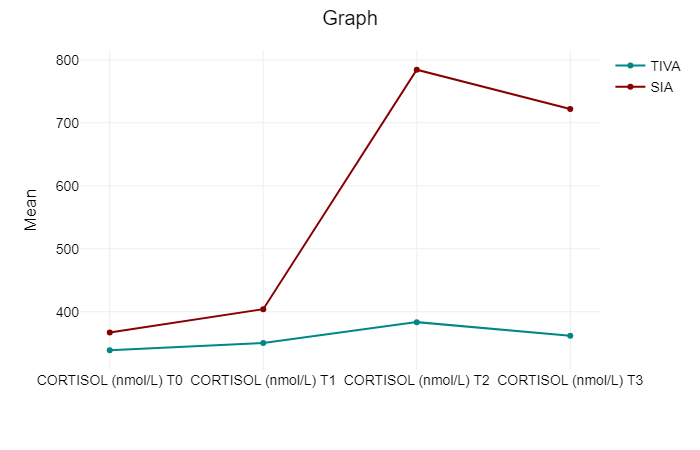
When comparing the cortisol levels at T0, both groups show a comparable range of variability. There is a highly significant difference in cortisol levels between TCI-TIVA and SIA following extubating, as indicated by the T2 p-value of less than 0.001. At T3, the pattern seen at T2 is still in place. Even 24 hours after surgery, the SIA group’s cortisol levels are noticeably higher than those of the TCI-TIVA group (Table 2) (Figure 2).
Table 2. Mean values of cortisol at different time points.
| CORTISOL (nmol/L) T0 | CORTISOL (nmol/L) T1 | CORTISOL (nmol/L) T2 | CORTISOL (nmol/L) T3 | Total | |
| TCI-TIVA | 338.85 | 350.19 | 383.5 | 361.95 | 358.62 |
| SIA | 366.98 | 404.03 | 784.25 | 721.85 | 569.28 |
| Total | 352.91 | 377.11 | 583.88 | 541.9 | 463.95 |
Figure 2. Trend of cortisol between the groups at different time point.
In the SIA group glucose levels remain relatively stable from T0 (5.29) to T1 (5.25), then increase slightly at T2 (6.56), and remain elevated at T3 (5.71). In the TCI-TIVA Group glucose levels show less variability, with a slight increase from T0 (5.17) to T2 (5.21) and remain steady at T3 (5.28) (Table 3).
Table 3. Comparison of glucose blood levels between SIA and TCI-TIVA.
| Type of Anesthesia | Glucose_T0 | Glucose_T1 | Glucose_T2 | Glucose_T3 |
| SIA | 5,29 | 5,25 | 6,56 | 5,7 |
| TCI-TIVA | 5,17 | 5,12 | 5,21 | 5,28 |
No significant difference is observed in glucose blood levels between the groups in T0 and T1. A significant difference is observed in glucose levels between the two groups after extubating (p < 0.001) and 24 hours after surgery. The TCI-TIVA group maintains more stable glucose levels compared to the SIA group (Figure 3).
Figure 3. Glucose blood levels (mmol/L) in T0, T1, T2 and T3.
There were notable differences in heart rate and mean arterial pressure (MAP) values between the groups. There were notable variations in heart rate (HR) during intubation (p <0.05), during the procedure (p <0.05), during extubating (p <0.05), and following extubating (p <0.05). HR values were greater in Group 2 (SIA) than in Group 1 (TCI-TIVA).
Systolic blood pressure, diastolic blood pressure and mean arterial pressure readings during intubation and extubating showed significant differences (p<0.05), with greater values observed in Group 2 (SIA).
Discussion
The regulation of inflammatory and stress reactions brought on by anesthesia and surgery is associated with a higher quality of recovery (16). Because propofol suppresses the release of cortisol and inflammatory mediators while blocking the generation of pro-inflammatory cytokines, it is thought to have anti-inflammatory and antioxidant effects (1,16,17). On the other hand, volatile anesthetics such as sevoflurane and isoflurane have been shown to increase pro-inflammatory cytokines, especially IL-6 and reduce neutrophil activity and lymphocyte proliferation (18). In order to mediate the acute phase response, IL-6 is essential. When an inflammatory stimulus is potent enough to have multiple systemic side effects that disrupt usual homeostatic processes, the result is an acute phase response of inflammation (19). In their study, Sofra M et al. observed a significant increase in IL-6 (6 – 8 hours after surgery) in both the TCI-TIVA and balanced inhalational anesthesia groups. The average value of this pro-inflammatory cytokine was 132pg/ml, indicating that the anesthetic method had no bearing on the increase in IL-6 (20). In contrast IL-6 was significantly higher in the inhalational group with isoflurane and fentanyl than in the TCI-TIVA group at the end of surgery, while these proinflammatory cytokines have not been statistically different values 12 hours after surgery (18). This is supported by the findings of Ihn CH et al. in which IL-6 levels were comparable at the various time points in both TCI-TIVA and SIA group. They noted intraoperative variations in IL-6 in the TCI-TIVA group, with a considerably lower level at intubation and a greater level at extubating when compared to baseline, while IL-6 in the SIA group was significantly elevated only at extubating time when compared with baseline (21). El Azab and colleagues, in their study on cardiac surgery with cardiopulmonary bypass noted significantly lower IL-6 in the group with TCI-TIVA than in the group with inhalational anesthesia prior to initiation of the procedure. However, there were no changes between the groups during or following the procedure (22). Our results are somewhat in agreement with the study of Ihc CH et al., we observed higher IL-6 levels at the time of extubating in both groups and the peak was 24 hours post-surgery with the TCI-TIVA group showing lower levels compared to the SIA group. This suggests that TCI-TIVA may better control the inflammatory response in the longer postoperative period. Concerning the neuroendocrine response, free cortisol in the blood decreases ACTH secretion and blocks CRH release in normal circumstances by acting as a negative feedback loop at the anterior pituitary and hypothalamic paraventricular nucleus. On the other hand, ultradian pulses in cortisol and ACTH significantly rise right after surgery. After then, within 24 hours, ACTH concentrations recover to normal in response to consistently high but less frequent cortisol pulses. Cortisol levels can stay elevated for seven days following surgery (1, 23). Propofol can reduce cortisol with a single induction dosage, but it cannot stop the release of cortisol and aldosterone as a reaction to surgical stress. During surgery, circulating cortisol secretion was totally eliminated by a continuous infusion of propofol at deep anesthetic dosages (24). The results in our study showed that cortisol levels were significantly lower in the TCI-TIVA group, suggesting that TCI-TIVA might be more effective in controlling the physiological stress response compared to SIA. Our results are comparable to the findings of Ozkan et al., Ihn CH et al., Mujagic et al and Onk D et al. Ozkan S et al., making a comparison of the effects of TCI-TIVA and sevoflurane anesthesia on the endocrine response in upper abdominal surgery and noticed lower cortisol concentration in the TCI-TIVA group postoperatively (25). Throughout the procedure, cortisol levels in the TCI-TIVA group stayed similar to baseline, whereas in the SIA group, levels were noticeably higher at intubation and extubating compared to baseline said Ihn CH et al. (21). Mujagic study’s findings demonstrated that patients treated under TCI-TIVA with propofol-fentanyl had significantly lower average blood levels of cortisol and prolactin intraoperative and shortly after surgery than patients treated under general balanced anesthesia with isoflurane-fentanyl. It is desirable, according to the data, that patients receiving TCI-TIVA had a weakened endocrine physiological response to stress during and immediately after surgery as in contrast to the inhalational group (7). TCI-TIVA was more effective in inhibiting cortisol release, which escalated due to the stress reaction, than inhalational anesthesia with desflurane in patients who underwent Coronary artery bypass surgery said Onk D in his study (26). Unlike our results, Soto et al. when comparing the glycemia, and cortisol levels at various time periods in their trial to baseline levels, neither the TCI-TIVA nor the inhalational group demonstrated any significant increases. They said that the hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis was effectively suppressed by both anesthetic methods (27). Also, no significant differences were noted between the TCI-TIVA and SIA group in the levels of catecholamines and cortisol in anterior resection of rectum in the study of Treda E et al. (28). Growth hormone, cortisol, glucagon, and catecholamines are “counter-regulatory” hormones whose levels rise in response to surgery or trauma. Their action leads to changes in the metabolism of carbohydrates and peripheral insulin resistance and results in hyperglycemia (29,30). Blood glucose levels were higher in the inhalational group with sevoflurane in the time of intubation, incision and extubating compared to TCI-TIVA group noted Ihn CH et al. (21). This was supported in the study of Mujagic et al., where they showed higher glucose levels in the group with isoflurane in the start of surgery, at the end and 2 hours after surgery, probably showing better attenuation of TCI-TIVA on the hormonal stress response (31). Increased glucose level was observed intraoperative in the inhalational groups with sevoflurane and isoflurane by Ozkan S et al. (25). Our findings are comparable to these, glucose blood level was significantly lower after extubating and postoperatively in the TCI-TIVA group. According to this, TCI-TIVA appears to be associated with a lower inflammatory response and more stable glucose metabolism, which could be beneficial for patient recovery. As opposed to these results, Soto et al. proved that during the procedure and for two hours afterward, the plasma glucose levels in both groups stayed constant (27). Considering the hemodynamic responses in our study, TCI-TIVA group experienced better hemodynamic stability. The SIA group had higher MAP and HR especially at intubation and extubating time. The studies by Ozkan S et al. and Juckenhöfel and coauthors showed the same outcomes. Significant increases in systolic and diastolic blood pressure were noted in the inhalational groups, particularly at intubation and extubating (25, 32). Similar results were observed in the Shah et al. study, where the heart rates of the sevoflurane group were significantly higher after surgery, but there was no significant difference in the heart rates of the two groups at intra-operative intervals, with the exception of 45 and 60 minutes (33). Similar results reported Ihc CH et al., with better control and consistency of blood pressure and heart rate in the TCI-TIVA group (21). In their spine surgery trial, He Lu et al. discovered that the TCI-TIVA group changed less in heart frequency and mean arterial pressure than the desflurane group. Moreover, the TCI-TIVA group’s heart rate and mean arterial pressure dropped during the extubating process (34). However, another study was not in agreement with our results. No significant differences between the groups in systolic and diastolic blood pressure were shown nor in the study of Soto et al., nor in the study of Lasinska-Kowara et al., though demonstrate hemodynamic stability in both groups equally (27, 35).
Other studies have demonstrated that during the post-surgical phase, women who are overweight (OW) or obese (OB) who have just been diagnosed with breast cancer (BC) have considerably higher levels of pro-inflammatory markers such interleukin-6 (IL-6) and interleukin-1β (IL-1β). Future research should focus more on OW/OB BC patients since they are a vulnerable category, as these elevated levels are linked to worse health outcomes. Nonetheless, according to our inclusion criteria, none of the patients’ BMIs exceeded 35kg/m2, and all patients’ BMIs fell within the normal range during our examination. As such, our study emphasizes the significance of comprehending how normal BMI patients respond to various anesthetic procedures in relation to their stress markers, such as IL-6, even if it did not particularly focus on the inflammatory response in OW/OB persons (36).
Further investigations are necessary to fully understand the overall stress response and hormone fluctuations in patients undergoing different anesthesia protocols. These studies should aim to explore the interplay between various stress markers and hormonal changes at different stages of surgery and recovery, providing a more comprehensive understanding of the physiological mechanisms involved and their potential impact on postoperative outcomes.
Conclusion
Our findings demonstrate that TCI-TIVA consistently outperforms the SIA group in terms of managing inflammation (IL-6) and the stress response (cortisol levels) before and after surgery. Furthermore, TCI-TIVA improves glucose levels and metabolic stability, especially during the crucial postoperative phase. These findings lend credence to the hypothesis that TCI-TIVA may perform superior to SIA in surgical settings where patient outcomes depend on lowering stress, inflammation, and metabolic issues.
References
- Cusack B, Buggy DJ. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020 Sep;20(9):321-328. doi: 10.1016/j.bjae.2020.04.006. Epub 2020 Jul 21. PMID: 33456967; PMCID: PMC7807970.
- Ivascu R, Torsin LI, Hostiuc L, Nitipir C, Corneci D, Dutu M. The Surgical Stress Response and Anesthesia: A Narrative Review. J Clin Med. 2024 May 20;13(10):3017. doi: 10.3390/jcm13103017. PMID: 38792558; PMCID: PMC11121777.
- Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr. 2013 Sep;37(5 Suppl):21S-9S. doi: 10.1177/0148607113496117. PMID: 24009246; PMCID: PMC3920901.
- Ivanovs, I., Mihelsons, M., & Boka, V. (2012). Stress Response to Surgery and Possible Ways of Its Correction. Proceedings of the Latvian Academy of Sciences. Section B. Natural, Exact, and Applied Sciences, 66(6), 225–233. doi:10.2478/v10046-012-0014-z
- Desborough, J. P. (2000). The stress response to trauma and surgery. Brit. J. Anaesth., 85 (1), 109–117.
- Lai HC, Lai MF, Huang YH, Yu JC, Tseng WC, Wu ZF. Comparison of Single Target-Controlled Infusion Pump-Delivered Mixed Propofol and Remifentanil with Two Target-Controlled Infusion Pumps-Delivered Propofol and Remifentanil in Patients Undergoing Breast Cancer Surgery-A Prospective Study. Int J Environ Res Public Health. 2023 Jan 23;20(3):2094. doi: 10.3390/ijerph20032094. PMID: 36767461; PMCID: PMC9915350.
- Mujagić, Z., Čičko, E., Vegar-Brozović, V., & Prašo, M. (2008). Serum levels of cortisol and prolactin in patients treated under total intravenous anesthesia with propofol-fentanyl and under balanced anesthesia with isoflurane-fentanyl. Open Medicine, 3(4). doi:10.2478/s11536-008-0051-9.
- Deng X, Zhu T. Clinical comparison of propofol-remifentanil TCI with sevoflurane induction/maintenance anesthesia in laparoscopic cholecystectomy. Pak J Med Sci. 2014 Sep;30(5):1017-21. doi: 10.12669/pjms.305.5196. PMID: 25225518; PMCID: PMC4163224.
- Nimmo AF, Absalom AR, Bagshaw O, Biswas A, Cook TM, Costello A, Grimes S, Mulvey D, Shinde S, Whitehouse T, Wiles MD. Guidelines for the safe practice of total intravenous anaesthesia (TIVA): Joint Guidelines from the Association of Anaesthetists and the Society for Intravenous Anaesthesia. Anaesthesia. 2019 Feb;74(2):211-224. doi: 10.1111/anae.14428. Epub 2018 Oct 31. PMID: 30378102.
- Vuyk J. Pharmacokinetic and pharmacodynamic interactions between opioids and propofol. J Clin Anesth. 1997 Sep;9(6 Suppl):23S-26S. doi: 10.1016/s0952-8180(97)00117-7. PMID: 9278851.
- Santonocito C, Noto A, Crimi C, Sanfilippo F. Remifentanil-induced postoperative hyperalgesia: current perspectives on mechanisms and therapeutic strategies. Local Reg Anesth. 2018 Apr 9;11:15-23. doi: 10.2147/LRA.S143618. PMID: 29670398; PMCID: PMC5898588.
- Delgado-Herrera L, Ostroff RD, Rogers SA. Sevoflurance: approaching the ideal inhalational anesthetic. a pharmacologic, pharmacoeconomic, and clinical review. CNS Drug Rev. 2001 Spring;7(1):48-120. doi: 10.1111/j.1527-3458.2001.tb00190.x. PMID: 11420572; PMCID: PMC6741648.
- Aranake A, Mashour GA, Avidan MS. Minimum alveolar concentration: ongoing relevance and clinical utility. Anaesthesia. 2013 May;68(5):512-22. doi: 10.1111/anae.12168. Epub 2013 Feb 16. PMID: 23414556.
- Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2014 Jun 17;2014(6):CD003843. doi: 10.1002/14651858.CD003843.pub3. Update in: Cochrane Database Syst Rev. 2019 Sep 26;9:CD003843. doi: 10.1002/14651858.CD003843.pub4. PMID: 24937564; PMCID: PMC6483694.
- Mathur S, Patel J, Goldstein S, et al. Bispectral Index. [Updated 2023 Nov 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539809/ .
- Kim DH, Min KT, Kim EH, Choi YS, Choi SH. Comparison of the effects of inhalational and total intravenous anesthesia on quality of recovery in patients undergoing endoscopic transsphenoidal pituitary surgery: a randomized controlled trial. Int J Med Sci. 2022 Jun 13;19(6):1056-1064. doi: 10.7150/ijms.72758. PMID: 35813289; PMCID: PMC9254366.
- Alhayyan A, McSorley S, Roxburgh C, Kearns R, Horgan P, McMillan D. The effect of anesthesia on the postoperative systemic inflammatory response in patients undergoing surgery: A systematic review and meta-analysis. Surg Open Sci. 2019 Jun 29;2(1):1-21. doi: 10.1016/j.sopen.2019.06.001. PMID: 32754703; PMCID: PMC7391900.
- Ke JJ, Zhan J, Feng XB, Wu Y, Rao Y, Wang YL. A comparison of the effect of total intravenous anaesthesia with propofol and remifentanil and inhalational anaesthesia with isoflurane on the release of pro- and anti-inflammatory cytokines in patients undergoing open cholecystectomy. Anaesth Intensive Care. 2008 Jan;36(1):74-8. doi: 10.1177/0310057X0803600113. PMID: 18326136.
- Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Analytic review: Interleukin-6 in surgery, trauma, and critical care: part I: basic science. J Intensive Care Med. 2011 Jan-Feb;26(1):3-12. doi: 10.1177/0885066610395678. PMID: 21262749; PMCID: PMC6209321.
- Sofra M, Fei PC, Fabrizi L, Marcelli ME, Claroni C, Gallucci M, Ensoli F, Forastiere E. Immunomodulatory effects of total intravenous and balanced inhalation anesthesia in patients with bladder cancer undergoing elective radical cystectomy: preliminary results. J Exp Clin Cancer Res. 2013 Feb 3;32(1):6. doi: 10.1186/1756-9966-32-6. PMID: 23374147; PMCID: PMC3577511.
- Ihn CH, Joo JD, Choi JW, Kim DW, Jeon YS, Kim YS, Jung HS, Kwon SY. Comparison of stress hormone response, interleukin-6 and anaesthetic characteristics of two anaesthetic techniques: volatile induction and maintenance of anaesthesia using sevoflurane versus total intravenous anaesthesia using propofol and remifentanil. J Int Med Res. 2009 Nov-Dec;37(6):1760-71. doi: 10.1177/147323000903700612. PMID: 20146874.
- El Azab SR, Rosseel PM, De Lange JJ, van Wijk EM, van Strik R, Scheffer GJ. Effect of VIMA with sevoflurane versus TIVA with propofol or midazolam-sufentanil on the cytokine response during CABG surgery. Eur J Anaesthesiol. 2002 Apr;19(4):276-82. doi: 10.1017/s0265021502000443. PMID: 12074417.
- Manou-Stathopoulou V, Korbonits M, Ackland GL. Redefining the perioperative stress response: a narrative review. Br J Anaesth. 2019 Nov;123(5):570-583. doi: 10.1016/j.bja.2019.08.011. Epub 2019 Sep 20. PMID: 31547969.
- Paola A, Carlo L, Cinzia DR, Valter P, Pierluigi N, et al. (2015) Stress Response to Surgery, Anesthetics Role and Impact on Cognition. J Anesth Clin Res 6: 539. doi:10.4172/2155-6148.1000539.
- Özkan, S., Yilmaz Cingözbay, B., Usyilmaz, S., Çankir, Z., Cebeci, B. S., & Gökben, M. (2001). Comparison of hemodynamic and neuroendocrine changes during total intravenous anesthesia and inhalation anesthesia. Current Therapeutic Research, 62(2), 142–152.
- Onk, Didem, Akarsu Ayazoğlu, Tülin, Onk, Oruç Alper, Aksüt, Mehmet, Günay, Murat, Turkmen, Kultigin, Özensoy, Aynur, Yazıcı Ersoy, Çiğdem, Çoban, Abdulkadir, Comparison of TIVA and Desflurane Added to a Subanaesthetic Dose of Propofol in Patients Undergoing Coronary Artery Bypass Surgery: Evaluation of Haemodynamic and Stress Hormone Changes, BioMed Research International, 2016, 3272530, 6 pages, 2016. https://doi.org/10.1155/2016/3272530.
- Soto, G., Pignolo, F., Calero, F., Saucina, F., Lainatti, L., Molinari, S. and Harvey, G. (2017) Evaluation of Stress Response and Apoptosis on Leucocytes in TIVA versus Balanced Anesthesia. Open Journal of Apoptosis, 6, 1-16. http://dx.doi.org/10.4236/ojapo.2017.61001.
- Wojarska, Elżbieta & Olejnik, Krzysztof & Stojcev, Zoran & Bialka, Szymon & Misiołek, Hanna. (2018). Choosing the optimal method of anesthesia in anterior resection of the rectum procedures – the assessment of the stress reaction based on selected hormonal parameters. Endokrynologia Polska. 69. 10.5603/EP.a2018.0038.
- Davis G, Fayfman M, Reyes-Umpierrez D, Hafeez S, Pasquel FJ, Vellanki P, Haw JS, Peng L, Jacobs S, Umpierrez GE. Stress hyperglycemia in general surgery: Why should we care? J Diabetes Complications. 2018 Mar;32(3):305-309. doi: 10.1016/j.jdiacomp.2017.11.010. Epub 2017 Nov 29. PMID: 29273446; PMCID: PMC5975368.
- Gupta B , Gupta A, Gupta L, Stress or metabolic response to surgery and anesthesia. Indian J Clin Anaesth 2019;6(2):165-171.
- Mujagić Z, Čičko E, Vegar-Brozović V, Prašo M. Serum levels of glucose and lactate in patients treated under total intravenous anesthesia with propofol-fentanyl and under balanced anesthesia with isoflurane-fentanyl. Biochem Med (Zagreb). 2007;17:71-78.
- Juckenhöfel S, Feisel C, Schmitt HJ, Biedler A. TIVA mit Propofol/Remifentanil oder balancierte Anästhesie mit Sevofluran/Fentanyl bei laparoskopischen Operationen. Hämodynamik, Aufwachverhalten und Nebenwirkungen [TIVA with propofol-remifentanil or balanced anesthesia with sevoflurane-fentanyl in laparoscopic operations. Hemodynamics, awakening and adverse effects]. Anaesthesist. 1999 Nov;48(11):807-12. German. doi: 10.1007/s001010050789. PMID: 10631440.
- Shah J, Varma N. Comparison of hemodynamic stability and recovery profile with sevoflurane as inhalational agent versus propofol as total intravenous anesthesia during laparoscopic surgeries. Anaesth Pain & Intensive Care 2018;22(2)212-218.
- Lu, C.-H., Wu, Z.-F., Lin, B.-F., Lee, M.-S., Lin, C., Huang, Y.-S., & Huang, Y.-H. (2016). Faster extubation time with more stable hemodynamics during extubation and shorter total surgical suite time after propofol-based total intravenous anesthesia compared with desflurane anesthesia in lengthy lumbar spine surgery. Journal of Neurosurgery: Spine, 24(2), 268–274. doi:10.3171/2015.4.spine141143.
- Łasińska-Kowara M, Kardel-Reszkiewicz E, Owczuk R. Zmiany parametrów hemodynamicznych podczas podtrzymywania znieczulenia propofolem lub sewofluranem [Effects of sevoflurane versus target-controlled infusion of propofol on haemodynamics during elective breast surgery in healthy women]. Anestezjol Intens Ter. 2009 Jul-Sep;41(3):135-9. Polish. PMID: 19999599.
- Ream M, Saez-Clarke E, Taub C, Diaz A, Frasca D, Blomberg BB, Antoni MH. Brief Post-Surgical Stress Management Reduces Pro-Inflammatory Cytokines in Overweight and Obese Breast Cancer Patients Undergoing Primary Treatment. Front Biosci (Landmark Ed). 2022 May 7;27(5):148. doi: 10.31083/j.fbl2705148.