Asani P1, Antovska V1, Stojchevski S1, Aluloski I1, Karadjova D2, Tanturovski M1
1University Clinic for Gynecology and Obstetrics-Skopje, Department of Gynecologic Oncology, Faculty of Medicine, University “Ss. Cyril and Methodius”, Skopje, Republic of North Macedonia
2University Clinic for Gynecology and Obstetrics-Skopje, Department of Anesthesia, Faculty of Medicine, University “Ss. Cyril and Methodius”, Skopje, Republic of North Macedonia
DOI: https://www.doi.org/10.55302/MJA2373015a
Abstract
Introduction: Endometrial cancer is the most common gynecological malignancy. In this study, the diagnostic accuracy of subjective methods with transvaginal ultrasonography for the assessment of deep myometrial and cervical invasion in patients with endometrial cancer was analyzed in order to choose the optimal surgical treatment.
Material and Methods: It represents a prospective cohort study in which 45 patients with a histological diagnosis of endometrial cancer were analyzed. They are examined with transvaginal ultrasound to assess deep myometrial and cervical invasion with subjective methods based on IETA (International Endometrial Tumor Analysis) terminology.
Results: Subjective assessment of deep myometrial invasion based on the International Endometrial Tumor Analysis (IETA) endometrial cancer terminology yielded a sensitivity of 88% and a specificity of 65%. Subjective assessment of deep myometrial invasion includes tumor echogenicity, endometrial-myometrial junction, color Doppler result and vascular pattern.Subjective assessment of cervical invasion includes loss of clear demarcation of tumor tissue from the cervical stroma and in the presence of stronger perfusion. Our subjective assessment of cervical invasion had a sensitivity of 76% and a specificity of 92%.
The obtained results are comparable with relevant works on this issue.
Conclusion: Transvaginal ultrasonography is similarly effective to nuclear magnetic resonance for assessing deep myometrial and cervical invasion; therefore, it can be used as a preoperative triage diagnostic tool to choose a surgical modality from which the mostly poor surgical candidate patients would benefit.
Key Words: cervical invasion, myometrial invasion, subjective methods, transvaginal ultrasound.
Introduction
Endometrial carcinoma is the most prevalent gynecological cancer in developed nations and the second most common in developing ones. The predominant histological type is endometrioid endometrial carcinoma, associated with a favorable prognosis, often presenting early with abnormal uterine bleeding. Conversely, other histologic types, such as serous, clear cell, carcinosarcoma, and various mixed uterine carcinomas, tend to have a poorer prognosis (1).
This cancer affects 1-2% of women in developed countries, with a peak incidence between ages 60 and 70, although a notable percentage occurs before age of 40. Chronic elevation of estrogen, without sufficient counteraction by progestin, is a primary risk factor, encompassing factors like tamoxifen use, clomiphene use, chronic anovulation, obesity, estrogen-secreting tumors and hormonal factors (1-7).
Less common type 2 neoplasms, characterized by nuclear grade 3 endometrioid histology and non-endometrioid histology, are not estrogen-sensitive, arise from atrophic endometrium, and have a less understood etiology(8). Lynch syndrome, Cowden syndrome, a family history of endometrial cancer and potentially BRCA mutations are hereditary factors which increase the risk (9-12).
Clinical presentation typically involves abnormal uterine bleeding in 75-90% of cases. However, bleeding quantity alone does not strongly correlate with the risk; factors like age and other risk factors should be considered. Diagnosis often occurs over the age of 55, through abnormal cervical cytology, incidental findings from various imaging modalities or during hysterectomy for other reasons.
Pelvic examination is less informative, and ultrasound in postmenopausal patients becomes suspicious when endometrial thickness approaches 20mm. An endometrial thickness below 4mm in premenopausal patients correlates with low risk. Diagnosis is established through dilatation and curettage, hysteroscopy or endometrial biopsy.
Endometrial cancers are traditionally divided into two types: type 1, representing 80% of the cases, is estrogen-driven, originating from endometrial hyperplasia, and has a good prognosis(13,14). Lack of exposure to progesterone is considered equally important in its pathogenesis. Type 2, clinically aggressive with histology like serous and clear cell, responds poorly to progesterone treatment and has a less favorable prognosis (13-15).
The newer molecular classification offers more consistent categorization with better predictive and prognostic information through genomic and proteomic analyses (16).
Prognostically, endometrial cancers are categorized into low-risk and high-risk. High-risk factors include poor differentiation, non-endometrioid histology, deep myometrial invasion, and tumors larger than 20mm on preoperative imaging. Treatment for high-risk cases involves radical surgery with lymph node evaluation. The low-risk endometrioid cancers with grades 1 and 2 and myometrial invasion <50% are treated with extrafascial hysterectomy without lymph node evaluation (17).
Objective
Utilizing transvaginal ultrasonography before surgery serves as a preoperative screening tool for patients diagnosed with endometrial cancer. The objective is to identify individuals at a heightened risk of deep myometrial and cervical invasion, aiding in the selection of appropriate treatment strategies. For those deemed at high risk, radical interventions involving parametrectomy and lymphadenectomy are necessary, ensuring optimal treatment. Conversely, patients identified as low risk through preoperative ultrasound would undergo extrafascial hysterectomy, avoiding the potential hazards associated with more extensive surgical procedures. This approach is particularly advantageous for elderly individuals, those with obesity, hypertension, diabetes and other comorbidities, where extensive surgical treatments could negatively impact overall prognosis and recovery. Ultrasound, serving as an alternative to nuclear magnetic resonance, offers comparable sensitivity and specificity, making it an ideal, cost-effective and easily applicable tool in routine clinical practice.
Material and Methods
A prospective cohort clinical study involved 45 patients diagnosed with endometrial cancer through procedures such as dilatation and curettage, hysteroscopy or endometrial biopsy. The study conducted at the University Clinic for Gynecology and Obstetrics included patients meeting the criteria and providing informed consent.
Each patient diagnosed with endometrial carcinoma underwent preoperative transvaginal ultrasound without prior knowledge of the histological subtype or nuclear grade. Patients’ selection adhered to specific inclusion and exclusion criteria.
Ultrasound examination was done by Voluson S6 General Electronics (GE), 2021.
Table 1. Demographic variables of 45 patients presented in numbers and percentage (n/%)
| Variables | The number and percentages of 45 patients |
| BMI kg/m2 of 45 patients | Normal weight 5/11%, Undernourished1/2%, Overweight15/33%, Obesity class 116/35%, Obesity class 21/2%, Obesity class 37/15% |
| Menopausal status of 45 patients | Reproductive period 9/20%, Menopause 36/80% |
| Current use of high/medium potential hormone therapy, 45 patients | Yes 43/95% No 2/4% |
| FIGO stage | 45 patients |
| IA | 20/44% |
| IB | 7/15% |
| II | 14/31% |
| IIIA | 0 |
| IIIB | 1/2% |
| IIIC1 | 3/6% |
| IIIC2 | 0 |
| IVA | 0 |
| IVB | 0 |
| Histological subtype and grade | 45 patients |
| Endometrioid adenocarcinoma G1 NG1 | 3/6% |
| Endometrioid adenocarcinoma G2 NG2 | 27/60% |
| Endometrioid adenocarcinoma G3 NG3 | 5/11% |
| Serous | 4/9% |
| Mixed | 4/9% |
| Carcinosarcoma | 2/4% |
Inclusion criteria:
- All patients with a diagnosis of endometrial cancer and atypical hyperplasia.
Exclusion criteria:
- Patients without hysterectomy or with a hysterectomy performed more than 120 days after ultrasound assessment.
- Final diagnosis other than endometrial cancer.
- Presence of tumor duplication or another synchronous gynecological malignancy.
- Incomplete ultrasound assessment.
- Refusal of signed consent to participate in the study.
Ultrasonography was conducted in a lithotomy position with an empty bladder. The ultrasound device initially zoomed in on the uterus in a mid-sagittal section for clearer visualization, evaluating the uterus from horn to horn (lateral to lateral wall) and in transverse cross-section from cranial to caudal. The subjective 2D assessment of myometrial and cervical invasion followed the International Endometrial Tumor Analysis IETA Terminology, considering tumor echogenicity, endometrial-myometrial junction, color Doppler result, and vascular pattern (Figure1).
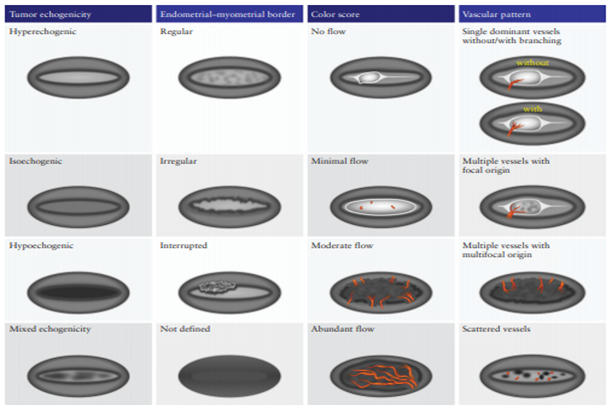
Figure 1. Schematic diagram summarizing morphological and Doppler features according to IETA terminology (18).
Tumor echogenicity can be isoechoic, hypoechoic, heterogeneous and hyperechoic.
Endometrial myometrial junction can be regular, irregular, interrupted or undefined.
Color Doppler: no flow, minimal flow, moderate flow, abundant flow.
Vascular pattern: single blood vessel with or without laceration, multiple blood vessels of focal origin, multiple blood vessels of multifocal origin, scattered blood vessels(Pictures 1-6).
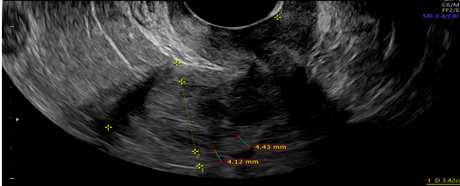
Picture1.Interrupted endometrial myometrial junction- green cursors.
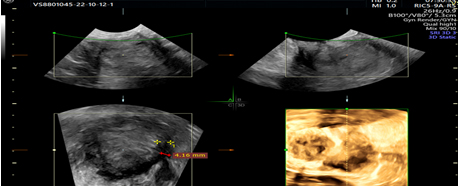
Picture 2. 3D ultrasonographic view of interrupted endometrial myometrial junction, green cursor.
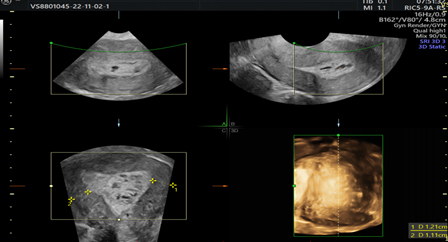
Picture 3. 2D and 3D ultrasonographic images with heterogeneous endometrium.
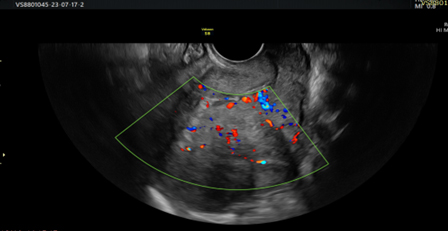
Picture 4. Doppler view of heterogeneous endometrium with interrupted endometrial myometrial junction and scattered blood vessels.
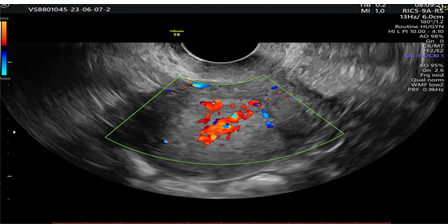
Picture 5.Prominent Doppler flow diagram.
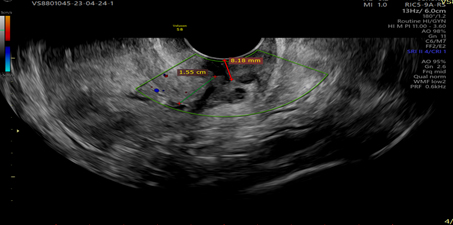
Picture 6.Showing tumor invasion into the cervical stroma, visualizes clear demarcation of tumor echogenicity from the cervical stroma.
Results
A total of 45 patients were included in this study, for which statistical data on sensitivity, specificity, positive predictive value, negative predictive value, prevalence of disease, positive likelihood ratio, negative likelihood ratio and accuracy were calculated for the subjective methods not listed above. All of them are comparedto the definitive postoperative pathohistology.
Table 2. Subjective deep myometrial invasion.
| Statistics | Value | 95% CI |
| Sensitivity | 88.00% | 68.78% to 97.45% |
| Specificity | 65.00% | 40.78% to 84.61% |
| Positive Likelihood Ratio | 2.51 | 1.36 to 4.65 |
| Negative Likelihood Ratio | 0.18 | 0.06 to 0.56 |
| Disease prevalence (*) | 55.56% | 40.00% to 70.36% |
| Positive Predictive Value (*) | 75.86% | 62.96% to 85.32% |
| Negative Predictive Value (*) | 81.25% | 58.84% to 92.93% |
| Accuracy (*) | 77.78% | 62.91% to 88.80% |
Table 3. Subjective cervical invasion.
| Statistics | Value | 95% CI |
| Sensitivity | 76.47% | 50.10% to 93.19% |
| Specificity | 92.86% | 76.50% to 99.12% |
| Positive Likelihood Ratio | 10.71 | 2.74 to 41.77 |
| Negative Likelihood Ratio | 0.25 | 0.11 to 0.60 |
| Disease prevalence (*) | 37.78% | 23.77% to 53.46% |
| Positive Predictive Value (*) | 86.67% | 62.49% to 96.21% |
| Negative Predictive Value (*) | 86.67% | 73.28% to 93.91% |
| Accuracy (*) | 86.67% | 73.21% to 94.95% |
Discussion
The staging process for endometrial cancer differs from that of cervical cancer, where clinical staging is employed. As the most prevalent gynecological neoplasm, endometrial cancer staging is donesurgically. The most patients diagnosed with endometrial cancer undergo a hysterectomy with bilateral salpingo-oophorectomy and retroperitoneal lymphatic dissection to determine the extent of the disease. Lymph node involvement rates increase with nuclear-grade progression and myometrial invasion. Therefore, lymph node evaluation is crucial, especially for patients meeting Mayo criteria, indicating type 2 endometrial carcinomas, nuclear grade 3 endometrioid endometrial carcinomas, neoplasms invading over half of the myometrium, or neoplasms exceeding 20mm on imaging or intraoperative assessment (17).
Patients not meeting Mayo criteria have a lymph node involvement risk of less than 5%, making lymph node dissection unnecessary reducing complications such as lymphedema, cellulitis and thrombotic events. This approach particularly benefits patients with advanced age, obesity, hypertension, diabetes and comorbidities, minimizingthe postoperative complications and improving long-term recovery and survival.
Subjective assessment using IETA terminology aids in distinguishing high-risk from low-risk endometrial cancers and is crucial in the prediction of myometrial and cervical invasion. Morphological differences between well, moderately, and poorly differentiated endometrioid carcinomas become apparent, with tumor size proving to be the only ultrasound predictor of high-risk cancer (18).
Studies comparing subjective and objective methods for myometrial and cervical invasion detection suggest that subjective assessments, particularly when utilizing IETA terminology, can be as effective as or even superior to objective measurements, such as that of F. MASCILINI, according to which subjective assessment of cervical and myometrial invasion is as good or better than any objective measurement technique (19).
The one of J. L. Alcázar reveals that the diagnostic potential of transvaginal ultrasonography in detecting deep myometrial invasions in women with endometrial cancer, gives a cumulative sensitivity of 82% and a cumulative specificity of 81%. Comparing subjective with objective measurement techniques,it has been observed that all methods are similar (20).
Filip Frühauf concluded that the sensitivity of the subjective assessment of myometrial invasion was superior to the investigated objective models (21).
In our subjective assessment of deep myometrial invasion based on the IETA terminology, the sensitivity and specificity are 88% and 65%, respectively (Table 2).
Various variables from the subjective and objective techniques for assessing myometrial and cervical invasion affect the accuracy of the results, leading to overestimation or underestimation. The accuracy of subjective assessment of myometrial and cervical invasion by ultrasound was significantly influenced by tumor size, tumor vascularization density, tumor vessel architecture and histological appearance. However, it was not significantly affected by BMI, uterine position and picture quality (22).
Subjective methods can also assess cervical invasion. Subjective assessment of cervical invasion may be more successful because dynamic ultrasonographic assessment can differentiate bulging or protrusion into an endocervical canal from actual cervical stromal invasion. Cervical stromal invasion is characterized by a loss of clear demarcation of the endometrial lesion from the cervical stroma and by increased tumor perfusion.
Our subjective assessment of cervical invasion had a sensitivity of 76% and a specificity of 92% (Table 3).
Regarding 3D Ultrasonography, there are different data regarding its effectiveness in predicting deep myometrial and cervical stromal invasion. Its advantages are that it can provide significant information about myometrial invasion of the uterine angles in a coronary section (23).
However, 2D ultrasonography is less effective than 2D ultrasonography for assessing myometrial invasion (23). Subjective assessment of myometrial invasion has a sensitivity of 92.6% and a specificity of 82.3% (24).
The possibilities of 3D ultrasonography, such as volume computerized imaging, computerizedultrasonographic tomography and rendering, can help assess myometrial and cervical invasion (Picture 3) (24).
Conclusion
Subjective assessment of deep myometrial and cervical invasion based on the IETA terminology is of great help; its sensitivity and specificity are close to, even better than, the previously known objective parameters for assessment of deep myometrial and cervical invasion. Our results are similar to other relevant publications in this field. Transvaginal ultrasonographyalso has accuracy similar to that of nuclear magnetic resonance.Subjective evaluation methods can significantly benefit patients’ selection of the optimal surgical modality, especially poor surgical candidates.
References:
- Brinton LA, Berman ML, Mortel R, et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J ObstetGynecol 1992; 167:1317.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice: Tamoxifen and endometrial Cancer. Committee opinion 232. ACOG 2000; Washington, DC.
- Zeleniuch-Jacquotte A, Akhmedkhanov A, Kato I, et al. Postmenopausal endogenous oestrogens and risk of endometrial cancer: results of a prospective study. Br J Cancer 2001; 84:975.
- Lukanova A, Lundin E, Micheli A, et al. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int J Cancer 2004; 108:425.
- Nyholm HC, Nielsen AL, Lyndrup J, et al. Plasma oestrogens in postmenopausal women with endometrial cancer. Br J ObstetGynaecol 1993; 100:1115.
- Potischman N, Hoover RN, Brinton LA, et al. Case-control study of endogenous steroid hormones and endometrial cancer. J Natl Cancer Inst 1996; 88:1127.
- Hacker NF, Friedlander ML. Uterine Cancer. In: Berek& Hacker’s Gynecologic Oncology, 7th ed, Berek JS, Hacker NF (Eds), Lippincott Williams & Wilkins, 2020. p.371.
- NCCN. Cancer risks in Lynch syndrome by gene compared to the general population. version 3.2019 https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf (Accessed on January 13, 2020).
- Heald B, Mester J, Rybicki L, et al. Frequent gastrointestinal polyps and colorectal adenocarcinomas in a prospective series of PTEN mutation carriers. Gastroenterology 2010; 139:1927.
- Riegert-Johnson DL, Gleeson FC, Roberts M, et al. Cancer and Lhermitte-Duclos disease are common in Cowden syndrome patients. Hered Cancer ClinPract 2010; 8:6.
- Pilarski R, Stephens JA, Noss R, et al. Predicting PTEN mutations: an evaluation of Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome clinical features. J Med Genet 2011; 48:505.
- Win AK, Reece JC, Ryan S. Family history and risk of endometrial cancer: a systematic review and meta-analysis. ObstetGynecol 2015; 125:89.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. GynecolOncol. 1983 Feb;15(1):10-7. doi: 10.1016/0090-8258(83)90111-7. PMID: 6822361.14.
- Felix AS, Weissfeld JL, Stone RA, et al. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control 2010; 21:1851. 17.
- Brinton LA, Felix AS, McMeekin DS, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. GynecolOncol 2013; 129:277. 5-6.
- Kandoth C, Schultz N, et al. Cancer Genome Atlas Research Network, Integrated genomic characterization of endometrial carcinoma. Nature 2013; 497:67.
- Mariani A, Webb MJ, Keeney GL, et al. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J ObstetGynecol 2000; 182:1506.
- Epstein, E et al. “Ultrasound characteristics of endometrial cancer as defined by International Endometrial Tumor Analysis (IETA) consensus nomenclature: prospective multicenter study.” Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecologyvol. 51,6 (2018): 818-828. doi:10.1002/uog.18909.
- Mascilini, F et al. “Evaluating myometrial and cervical invasion in women with endometrial cancer: comparing subjective assessment with objective measurement techniques.” Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology vol. 42,3 (2013): 353-8. doi:10.1002/uog.12499.
- Alcázar, J L et al. “Transvaginal ultrasound for preoperative assessment of myometrial invasion in patients with endometrial cancer: a systematic review and meta-analysis.” Ultrasound in obstetrics &gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology vol. 46,4 (2015): 405-13. doi:10.1002/uog.14905.
- Frühauf F, Zikan M, Semeradova I, et al. The Diagnostic Accuracy of Ultrasound in Assessment of Myometrial Invasion in Endometrial Cancer: Subjective Assessment versus Objective Techniques. Biomed Res Int. 2017;2017:1318203. doi: 10.1155/2017/1318203. Epub 2017 Jul 24. PMID: 28812010; PMCID: PMC5546069.
- Fischerova, D et al. “Factors affecting sonographic preoperative local staging of endometrial cancer.” Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology vol. 43,5 (2014): 575-85. doi:10.1002/uog.132487.
- Christensen, Julie W et al. “Assessment of myometrial invasion in endometrial cancer using three-dimensional ultrasound and magnetic resonance imaging.” Actaobstetricia et gynecologicaScandinavica vol. 95,1 (2016): 55-64. doi:10.1111/aogs.12806.
- Alcázar, Juan Luis et al. “Assessing myometrial infiltration by endometrial cancer: uterine virtual navigation with three-dimensional US.” Radiology vol. 250,3 (2009): 776-83. doi:10.1148/radiol.2503080877.