Naumovski F1, Dimovska M2
1University Clinical Campus “Mother Theresa”, University Clinic for Traumatology, Orthopedics, Anesthesia, Resuscitation, Intensive Care and Emergency Center –Skopje
2Clinical Hospital “Acibadem Sistina”
UDK: 616.136-007.64:616.12-073.432.19-089.163
https://www.doi.org/10.55302/MJA2481070n
Abstract
This case presents highlights of the role of intraoperative echocardiography in the successful management of a 65-years-old male scheduled for abdominal aortic aneurysm (AAA) repair. The patient was presented with an abdominal aortic aneurysm with multiple comorbidities and recent myocardial infarction with post stenting hemopericardium, making the anesthetic management for the surgical intervention challenging. Intraoperative echocardiography played a crucial role in establishing decisions about the treatment and guiding therapy for ensuring a favorable outcome. This report emphasizes the significance of intraoperative echocardiography as a real time monitor of hemodynamics in enhancing patient’s safety and favorable clinical outcomes during high-risk surgical procedures.
Key Words: Abdominal Aortic Aneurism Repair, Intraoperative echocardiography. Point of care, Ultrasound.
Introduction
Abdominal aortic aneurysm (AAA) is a serious vascular condition characterized by the aortic wall weakening and dilation of the abdominal aorta greater than 3cm (1). AAA frequently demands surgical repair which timing depends on aneurism dimensions and symptomatology. If the AAA is left untreated, it poses a high risk of rupture, leading to life-threatening hemorrhage with possible death. AAA is related to significant cardiovascular morbidity and mortality, while most of the patients could be asymptomatic until rupture occurs when the overall mortality is up to 80% (2,3). Surgical intervention is often required to prevent rupture and maintain patient’s well-being. Some studies have shown that perioperative mortality for AAA surgical repair is 4-8%, while developing a major perioperative adverse event including myocardial infarction, pneumonia, cerebrovascular ischemia, kidney injury, deep vein thrombosis and colonic ischemia could be met in stunning 15-30% of the cases (4) making this intervention not only surgical, but rather a bigger challenge for the anesthesiologist. Therefore, AAA repair in complex cases demands careful anesthetic evaluation and vicious perioperative management, particularly when patients present with concurrent cardiovascular issues.
Case Presentation
A 65-years-old male with a significant medical history of hypertension, a heavy smoking habit (2 packs per day) and a recent diagnosis of acute coronary infarction was scheduled for AAA repair. Preoperative imaging revealed an infrarenal abdominal aortic aneurysm measuring 11cm in length and 4.5cm in diameter, with circumferential mural thrombosis and peripheral hyperdensity, indicating a high risk of rupture. Fourteen days before surgery, the patient complained of chest pain and breathing difficulties, prompting an electrocardiogram revealing occurrence of an acute myocardial infarction demanding coronary angiography and subsequent stenting. The angiography revealed severe stenosis in the left anterior descending artery (95%) and left main (99%), which necessitated the placement of two stents. Unfortunately, a coronary artery rupture occurred during the stenting, and the patient has developed a subsequent hemopericardium. Echocardiography has demonstrated pericardial effusion and presence of fresh coagulum inside pericardial sac, warranting further evaluation. A second coronary angiography the next day showed no extravasation of contrast, but the pericardium was observed to be thickened and filled with blood without any influence of the cardiac filling and contractility. The patient was treated with Clopidogrel 75mg and Aspirin 100mg due to coronary artery stenting, but because of the present abdominal pain and CT findings showing a high risk of AAA rupture, he was scheduled for a AAA surgical repair. High-risk informed consent due to possible anesthetic and surgery related complications was obtained from the patient prior to intervention. Due to the risk of stent thrombosis dual antiaggregating therapy was sustained even at the day of surgery. The anesthetic management of this complex case was made under standard noninvasive and invasive measurement of arterial blood pressure after right radial arterial line placement, which was inserted after a successful Allen’s test, as well as central venous pressure measurement after right jugular central line insertion using ultrasound. Vital signs before surgery showed hemodynamic instability with hypotension (90/50mmHg), a heart rate of 120bpm and oxygen saturation of 93%. The patient was started on a noradrenaline infusion (8mg/50ml) at a rate of 0.1mcg/kg/min to address the hypotension. Induction in anesthesia was made with a careful titrating of anesthetic medications using Midazolam 1mg, Ketamine 100mg, Propofol 20mg, Fentanyl 50mcg and muscle relaxation was provided by Cisatracurium 10mg. After induction and successful placement of an endotracheal tube with No. 8.5, the patient was ventilated using a pressure-control volume-guaranteed mode. Intraoperatively, anesthesia was maintained with sevoflurane (1% with MAC 0.5-0.7) while analgesia was provided by continuous infusion of remifentanil (2mg/50ml) at 0.05mcg/kg/min, Metamizole Sodium 2.5g and Acetaminophen 1g. Because of sustained hemodynamic instability with hypotension and tachycardia with heart rate of 120-130 beats per minute and requirement of escalating doses of continuous Norepinephrine infusion, we have made an intraoperative transthoracic echocardiography. Due to surgical isolation just below the fourth rib transthoracic examination was difficult and incomplete, but it has provided significant data for the hemodynamics of the patient leading us to a substantial change in intraoperative treatment. Actually, the echocardiographic findings have shown persistence of a pericardial effusion mentioned before without influence of right ventricular filling nor systolic function which ruled out the diagnosis of pericardial tamponade as a cause of hemodynamic instability (Figure 1). Echocardiographic examination has shown presence of atrial fibrillation with inappropriate left ventricular contraction seen by eyeballing and confirmed in M-mode tracing of the left ventricle. Also, absence of the atrial kick on transmitral doppler was confirmed which is considered as a pathognomonic sign for AFF. Left atrial enlargement was met without any thrombotic masses in the left heart. After the echocardiographic examination, the patient has received Metoprolol 5mg in slow i.v. bolus which resulted in pharmacological cardioversion leading to converting the patient into sinus rhythm with HR of 65 and hemodynamic stabilization with SBP of >120mmHg and DBP >70 and MAP greater than 65mmHg, in order to maintain adequate perfusion. Another quick point of care – echocardiographic evaluation was made later on which has confirmed conversion of atrial fibrillation into sinus rhythm with clear distinction of systole and diastole on the left ventricular M-mode tracing, and significant improvement of the cardiac output due to establishment of left ventricular competence (Figure 2). After pharmacological rhythm conversion into sinus rhythm, improvement of hemodynamics was met, and we have lowered the intraoperative doses of norepinephrine to 0.02mcg/kg/min. During the surgery we have used cell salvage in order to minimize blood loss and maintain hemodynamic stability. The surgery has lasted for 5 hours, with an aortic clamping time of approximately one hour and 10 minutes. After successful performance of surgical technique and fulfillment of extubation criteria, the patient was safely extubated in the operating room and transferred to the Post Anesthesia Care Unit for further monitoring. Prior to transport I PACU, he was completely hemodynamically stable without need of any vasopressors nor inotropes. Postoperatively the patient has received metoprolol once daily with no adverse events until hospital discharge.
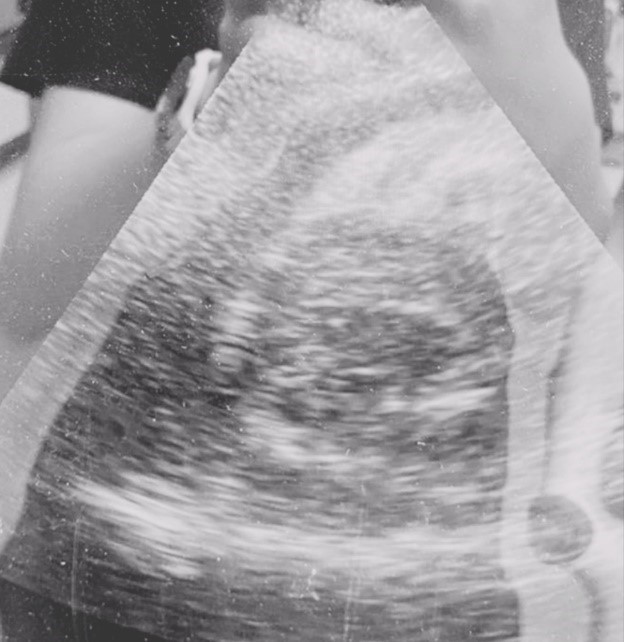
Figure 1. Pericardial effusion without impairment of ventricular filling.
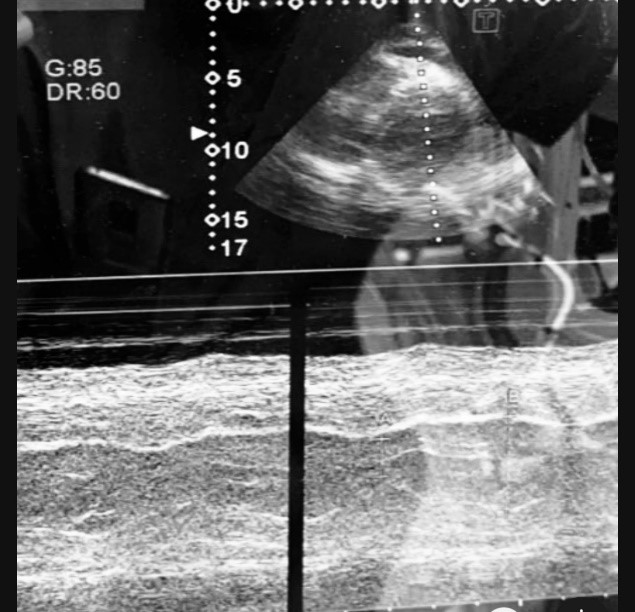
Figure 2. M-mode tracing of left ventricle with visible systolic and diastolic movement of the myocardium after cardioversion.
Discussion
Anesthetic management of patients with AAA with cardiovascular comorbidities, especially in semi-elective or in an emergent case could be a real challenge for the anesthesiologist. We report such a case with a recent acute cardiovascular accident making the case even more complicated. Because of the case complexity, as well as because of the risk of well-known possible intraoperative complications, we have used standard noninvasive and invasive monitoring due to picturing hemodynamics in a real time. It was well recognized before, that operative blood loss, metabolic and hemodynamic changes, induction of anesthesia, aortic clumping as well as declamping could cause substantial myocardial stress and lead to cardiac injury causing adverse events intraoperatively or in the early postoperative period (5). The negative effect over myocardial contractility provoked with aortic clamping during open aortic repair was described by Kalman et al. in their study implying that this crucial event of the surgery could influence myocardial performance by lowering cardiac contractility, which was proven to be returned to its baseline values after declamping (6). Diastolic compliance decrease related to aortic declamping was described also in the same study relaying to the echocardiographic measurements suggesting possible myocardial ischemic injury and ventricular dysfunction during the surgery (6). As it was mentioned in the previous study, Cuypers PW et al. have also identified that open aneurismatic repair by itself is a serious risk factor for developing intraoperative and perioperative ischemia (7). Using echocardiography, they have identified that aortic clamping and declamping are two critical events precipitating significant hemodynamic changes that could lead to myocardial injury and disfunction. Aortic declamping was identified as a critical event that could lead to Cardiac Index increasement due to lowering the SVR and elevating the preload with possibly deleterious effect in patients with previously established diagnosis of Coronary Artery Disease (CAD), due to higher myocardial metabolic activity and elevated oxygen demand (7). The presence of a multivessel CAD and the left main artery occlusion involvement were identified as risk factors for developing intraoperative myocardial ischemia, as well as postoperative myocardial ischemia in patients who underwent open AAA repair (8). Another study has highlighted the importance of CAD regarding mortality in patients undergoing open AAA repair, as well as the fact that correction of the severe CAD before surgery is beneficial in preventing perioperative mortality (9). Keeping in mind all above mentioned facts about the complexity of the surgical procedure regarding its hemodynamic effects and already present multivessel CAD, the occurrence of intraoperative and perioperative adverse cardiac events was more than likely. Despite the recent percutaneous intervention and correction of the severe CAD in our patient, which was previously stated as a beneficial in such a case, we have continued to be vigilant because of the acute myocardial infarction recentness and its influence over still not-healed cardiomyocytes. Because of all above mentioned risk factors, expected intraoperative complications, as well as well-known intraoperative hemodynamic changes, we have used transthoracic echocardiography as a real time, point of care monitor intraoperatively during diagnosis and treatment of hemodynamic instability. Most of the knowledge gained about the hemodynamic changes and myocardial performance during open AAA repair were gained by using transesophageal and transthoracic echocardiography (5-7). In our case due to lack of transesophageal echocardiography machine, we were forced to use transthoracic echocardiography in a very limited window due to surgical isolation. Nowadays, evaluation of cardiac function, competence and hemodynamics with a point of care echocardiography is strongly recommended by the European Society of Intensive Care Medicine. According to the recommendations, the evaluation of the left ventricular systolic, diastolic function and pericardial bed in cases of shock and hemodynamic instability is strongly advised and recommended to be done bedside (10) as in our case. In our case presentation, the usage of Transthoracic echocardiography (TTE) led to point of care diagnosis of cause of hemodynamic instability, leaving all presumptive differential diagnosis behind and has provided suitable guidance of therapy inside intraoperative period. Performing echocardiography and its benefits regarding atrial fibrillation was recommended by Shakya S et al. and Troughton RW et al. (11,12). Despite non-invasive and invasive monitoring during open AAA repair as a standard procedure, we have added TTE as a real time window in order to guide, maintain and correct hemodynamics, due to safely managing such a high-risk patient.
Conclusion
In this case presentation, we emphasize the crucial role of intraoperative echocardiography in guiding interventions and ensuring a favorable outcome for patients undergoing complex open abdominal aortic aneurysm repair. Performing a TTE has allowed prompt identification of the cause of hemodynamic instability and management of cardiac complications during surgery, contributing to greater safety and improved clinical outcomes. In absence of TEE equipment, even limited window TTE could provide significant real time data about hemodynamical happenings essential for maintaining hemodynamic stability. In high-risk surgeries, we recommend integrating intraoperative echocardiography as part of standard care protocol due to significant enhancement of patient’s care and optimizing surgical outcomes.
References:
- Schanzer A, Oderich GS. Management of Abdominal Aortic Aneurysms. N Engl J Med. 2021 Oct 28;385(18):1690-1698. doi: 10.1056/NEJMcp2108504. PMID: 34706173.
- Gao JP, Guo W. Mechanisms of abdominal aortic aneurysm progression: A review. Vasc Med. 2022 Feb;27(1):88-96. doi: 10.1177/1358863X211021170. Epub 2021 Jul 18. PMID: 34278882.
- Kuivaniemi H, Ryer EJ, Elmore JR, Tromp G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev Cardiovasc Ther. 2015;13(9):975-87. doi: 10.1586/14779072.2015.1074861. PMID: 26308600; PMCID: PMC4829576.
- Chaikof EL, Brewster DC, Dalman RL, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009;50(4 Suppl): S2–49.
- Falk JL, Rackow EC, Blumenberg R, Gelfand M, Fein IA. Hemodynamic and metabolic effects of abdominal aortic crossclamping. Am J Surg. 1981 Aug;142(2):174-7. doi: 10.1016/0002-9610(81)90270-1. PMID: 7258523.
- Kalman PG, Wellwood MR, Weisel RD, Morley-Forster PK, Teasdale SJ, Ivanov J, Johnston KW, McLaughlin PR, Baird RJ, Cain JP, et al. Cardiac dysfunction during abdominal aortic operation: the limitations of pulmonary wedge pressures. J Vasc Surg. 1986 May;3(5):773-81. PMID: 3701940.
- Cuypers PW, Gardien M, Buth J, Charbon J, Peels CH, Hop W, Laheij RJ. Cardiac response and complications during endovascular repair of abdominal aortic aneurysms: a concurrent comparison with open surgery. J Vasc Surg. 2001 Feb;33(2):353-60. doi: 10.1067/mva.2001.103970. PMID: 11174789.
- Blombery PA, Ferguson IA, Rosengarten DS, Stuchbery KE, Miles CR, Black AJ, Pitt A, Anderson ST, Harper RW, Federman J. The role of coronary artery disease in complications of abdominal aortic aneurysm surgery. Surgery. 1987 Feb;101(2):150-5. PMID: 3810485.
- Golden MA, Whittemore AD, Donaldson MC, Mannick JA. Selective evaluation and management of coronary artery disease in patients undergoing repair of abdominal aortic aneurysms. A 16-year experience. Ann Surg. 1990 Oct;212(4):415-20; discussion 420-3. doi: 10.1097/00000658-199010000-00004. PMID: 2222012; PMCID: PMC1358270.
- Robba C, Wong A, Poole D, Al Tayar A, Arntfield RT, Chew MS, Corradi F, Douflé G, Goffi A, Lamperti M, Mayo P, Messina A, Mongodi S, Narasimhan M, Puppo C, Sarwal A, Slama M, Taccone FS, Vignon P, Vieillard-Baron A; European Society of Intensive Care Medicine task force for critical care ultrasonography*. Basic ultrasound head-to-toe skills for intensivists in the general and neuro intensive care unit population: consensus and expert recommendations of the European Society of Intensive Care Medicine. Intensive Care Med. 2021 Dec;47(12):1347-1367. doi: 10.1007/s00134-021-06486-z. Epub 2021 Oct 5. PMID: 34787687; PMCID: PMC8596353.
- Shakya S, Gajurel RM, Poudel CM, Shrestha H, Devkota S, Thapa S. Echocardiographic Findings in Patients with Atrial Fibrillation in a Tertiary Care Center of Nepal: A Descriptive Cross-sectional Study. JNMA J Nepal Med Assoc. 2021 Jan 31;59(233):46-50. doi: 10.31729/jnma.5408. PMID: 34508458; PMCID: PMC7893398.
Troughton RW, Asher CR, Klein AL. The role of echocardiography in atrial fibrillation and cardioversion. Heart. 2003 Dec;89(12):1447-54. doi: 10.1136/heart.89.12.1447. PMID: 14617563; PMCID: PMC1767994.