UDK: 616.831-006.484-089.87-089.5
https://www.doi.org/10.55302/MJA2484123a
Adramanova D1, Stolevski V2,3, Abbas N1, Strezoska M1, Dimovski A2, Jakimoska Dimkovska M2
1 Department of Anesthesiology and Intensive Care, University Clinic for Surgical Diseases “St. Naum Ohridski” Skopje
2 Department of Neurosurgery, University Clinic for Surgical Diseases “St. Naum Ohridski” Skopje
3 University “Goce Delchev” Shtip
Abstract
The awake craniotomy, as a surgical procedure, facilitates maximal resection of brain tumors with minimal or no damage to eloquent brain areas. Conscious sedation and asleep-awake-asleep techniques are two anesthetic techniques employed. During the awake phase, the patient should be alert and cooperative, with adequate analgesia as an essential component of the anesthetic management while neurological testing is performed. Ensuring airway patency, proper oxygenation and ventilation, avoidance of hypoxia and hypercarbia, and maintaining stable systemic and cerebral hemodynamics are crucial. We present a case of a right-handed 56-years-old man with speech difficulties manifested as fluent dysphasia and magnetic resonance findings of intra-axial supratentorial left-sided temporoparietal lesions. The patient was scheduled for awake brain surgery according to the affected eloquent language brain area of the dominant hemisphere. Asleep-awake-asleep anesthetic technique was utilized. Short-acting sedative agents propofol and dexmedetomidine were used, along with remifentanil and fentanyl as analgesics. After awakening, neurological testing of sensory, motor and cognitive functions was performed, revealing no new neurological deficits and achieving maximally safe microsurgical tumor resection with stable hemodynamics and no respiratory or intracranial adverse events. With histopathological findings of glioblastoma (WHO Grade IV), chemotherapy and radiotherapy followed the surgery. The available literature demonstrates the importance of the extent of tumor resection for improved outcomes and survival benefits in glioma surgery, with maximal resection without excising functional brain tissue, being the primary goal of awake brain surgery.
Key Words: Asleep-awake-asleep technique; Awake brain surgery; glioblastoma.
Introduction
In 1886, the first awake craniotomies were performed by Horsley under local anesthesia for epilepsy surgery (1). Surgical treatment for supratentorial tumors, vascular malformations and any other lesion located near eloquent areas of the brain, can be facilitated using awake craniotomy (2). The awake craniotomy is a neurosurgical procedure in which the patient is awake for neurological testing during a whole procedure or a part of it. The brain cortex responsible for all functions we can examine is the eloquent cortex, like the motor and language cortex, so any supratentorial intra-axial lesion under the eloquent cortex could be considered (3). The awake craniotomy as a procedure is frequently considered in the surgical treatment of different types of gliomas, so maximal reduction of the tumor can be done while minimizing damage to the eloquent areas of the brain. Providing adequate analgesia and anxiolysis, systemic and cerebral hemodynamic stability, establishing airway patency and proper ventilation, as well as patient’s cooperation are challenges for the anesthesiologist. In general, there are two anesthetic techniques for this procedure: conscious sedation and asleep–awake–asleep technique.
Case Presentation
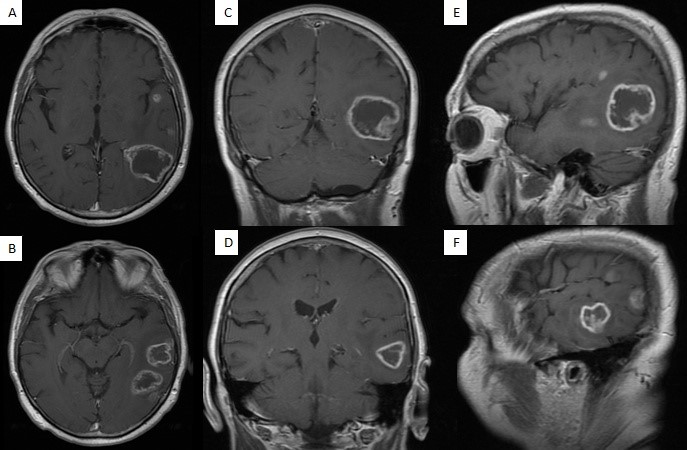
A 56-years-old right-handed male patient presented to the neurosurgery department with a three-weeks history of speech difficulties manifested as fluent dysphasia. While working as an elementary school teacher, the patient initially noted the speech disturbances manifested as word-retrieval difficulties (e.g., he wants to say a word/ phrase but can’t recall it). Afterward, his spouse noticed the speech difficulties, described as problems with counting, basic arithmetic skills and word retrieval difficulties. The complaints mentioned above gradually worsened over the past three weeks. The neurological assessment indicated fluent dysphasia,- mainly anomic, with no other neurological deficits. A contrast-enhanced brain MRI revealed ring-enhancing intra-axial supratentorial temporoparietal left-sided lesions (Figure 1). Two of the five abovementioned lesions were of greater diameter, with peritumoral vasogenic edema, thick walls, and a core area with a reduced signal (Figure 1), proposing multifocal glioblastoma as the most probable diagnosis.
Figure 1. Contrast-enhanced brain MRI.
A, B – axial T1 sequences – two greater diameter ring-enhancing lesions of surgical interest
(greatest diameters of 25.3mm – temporal and 41mm – temporoparietal, respectively).
C, D – coronal T1 sequences. E, F – sagittal T1 sequences.
Surgical treatment was initially planned to achieve maximum safe tumor resection and histopathological verification. Subsequently, after further in-depth evaluation, the patient was scheduled for awake brain surgery according to the affected eloquent language brain area of the dominant hemisphere, neurological examination findings, the need for intracranial decompression and maximal safe resection, and histopathological verification for further treatment planning. A preoperative assessment by an anesthesiologist was done, and consent was taken from the patient after giving information about the procedure. The patient was without significant medical history. Anticonvulsive therapy with carbamazepine was initiated after the diagnosis of the tumor, along with intravenous dexamethasone and mannitol, before the surgery. The asleep-awake-asleep anesthesia technique was utilized (Figure 2). After standard non-invasive and invasive blood pressure monitoring, the patient was induced in general anesthesia with midazolam and propofol, fentanyl as opioid analgetic, and muscle relaxation with rocuronium. Mechanical ventilation was initiated after the insertion of the endotracheal tube. The patient was positioned in a semi-lateral position (left shoulder elevated) with the head rotated around 45 degrees to the right and placed on a horseshoe headrest, with protection of all bony prominences and nerves. After sterile skin preparation and draping of the surgical field, the scalp was infiltrated along with the planned incision with local anesthetic lidocaine. He underwent a left-sided temporoparietal craniotomy, and microsurgical maximally safe resection of the two (out of five) larger diameter lesions (Figure 1) was performed (Figure 2 – C). General anesthesia was maintained with propofol in continuous infusion (50–70μg/ kg/min), and analgesia was provided with boluses of fentanyl and remifentanil infusion (0.075 – 0.15μg/kg/min). Later, dexmedetomidine infusion was added in doses of 0.5-0.7μg/kg/h after a bolus dose of 0.5μg/kg over 10 minutes. Fifteen minutes before the patient’s planned awakening, propofol infusion was discontinued. Dexmedetomidine infusion in lower doses (0.2μg/kg/h) and remifentanil (0.01 – 0.02μg/kg/min) remained for maintaining proper analgesia and anxiolysis through an awake phase.
Shortly after, the patient was awakened when his name was called loudly. He was cooperative, without pain, and had stable systemic hemodynamics. The endotracheal tube was removed, with good oxygen saturation and airway patency. Continuous intraoperative assessments of language, sensory, cognitive and motor functions were performed, and all the examined functions were preserved entirely (identical to preoperative assessments) while achieving maximal safe tumor resection (Figure 2-B). Again, general anesthesia was induced for hemostasis and closure of the skull. The endotracheal tube was reinserted with video laryngoscopy, and ventilation was controlled. No adverse events occurred, such as airway obstruction, hemodynamic instability, raised intracranial pressure, intracranial hemorrhage, or seizures. He was discharged on the ninth postoperative day with fluent dysphasia, which was present at admission, without any new neurological deficits.
The histopathology findings revealed tumor proliferation built by atypical glial cells (protoplasmic and fibrillary astrocytes with gemistocytes), cellular and nuclear atypia, bizarre giant cells and bizarre nuclei. Furthermore, newly formed blood vessels with marked endothelial proliferation, coagulative palisade tumor necrosis, and intratumoral hemorrhage fields were noted. The described morphology was consistent with glioblastoma (WHO Grade IV). Further treatment continued at the oncology clinic, where he underwent postoperative chemotherapy and radiotherapy, and regular follow-ups were scheduled and performed.
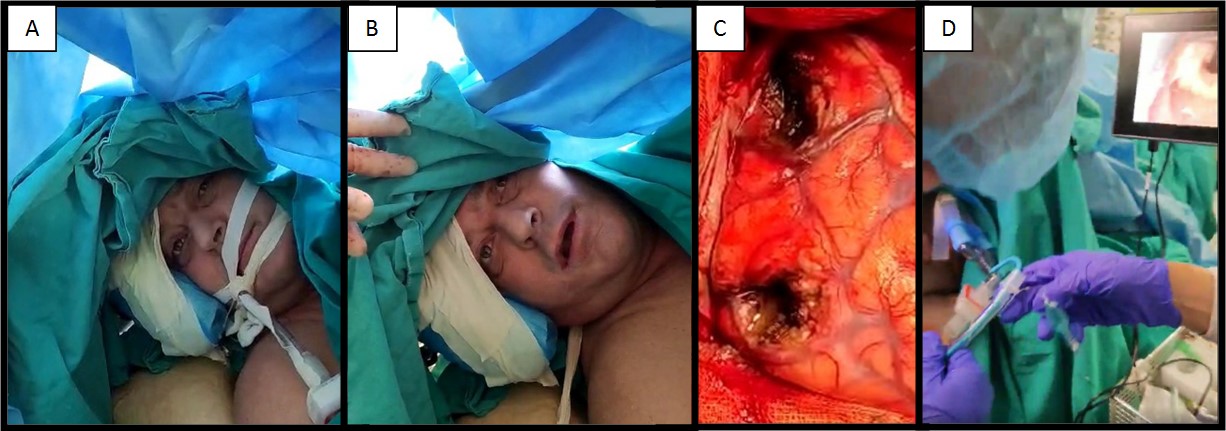
Figure 2. Awake brain surgery (asleep-awake-asleep technique) – Intraoperative photographs (approved with patient’s consent).
A – view of the initial part of the asleep-awake anesthesia technique.
B – intraoperative assessment of language/ motor functions (awake part).
C – Microsurgical maximal safe tumor resection of two larger-diameter lesions via temporoparietal craniotomy.
D – reintubation utilizing video laryngoscopy (asleep part).
Discussion
The available literature demonstrates the importance of the extent of tumor resection for improved outcomes and survival benefits in glioma surgery, with maximal resection without excising functional brain tissue being the primary goal of awake brain surgery (4-10). An extensive literature review shows that the indications for awake surgery for glioma patients include assessment of language functions, sensorimotor pathways, visuospatial pathways and executive functions (11). Generally, the main indications for awake brain surgery include resection of tumors in eloquent brain areas such as Brodmann areas 1,2,3,4, language cortex (Broca’s and Wernicke’s area), and motor eloquent area (8).The current standard of care for glioblastoma encompasses maximally safe microsurgical resection and following chemotherapy and radiotherapy (12). Awake craniotomy for glioblastoma removal in eloquent areas leads to fewer neurological deficits, improved overall survival, and longer progression-free survival while maintaining quality of life. This highlights the safety and feasibility of the procedure, along with its positive outcomes (13, 14).
Choosing suitable patients is crucial for this procedure’s success and prevents perioperative complications. Because of the necessity for patient cooperation, individuals with somnolence, drowsiness, confusion, altered mental status, psychiatric disorders and severe dysphasia, should be considered carefully (3). Ensuring adequate oxygenation and ventilation during all phases of awake surgery may be difficult for patients with morbid obesity, especially those with obstructive sleep apnea. Along with the anticipated difficult airway, these factors present relative contraindications (15). In patients with uncontrolled coughing, increased intracranial pressure and uncontrolled seizures, there is also a relative contraindication for awake craniotomy (16). Patient cooperation is essential; therefore, declining the procedure after a full explanation is an absolute contraindication.
The awake craniotomy, a procedure used in supratentorial brain tumor surgery, allows for neurological testing and facilitates maximal tumor excision with minimal damage to functional brain tissue. A literature review from PubMed and Medline databases showed shorter hospital stays, slightly less mean extent of resection, and slightly less surgery time in awake craniotomy compared to craniotomy under general anesthesia (17). Postoperative neurological deficits were less frequent with awake craniotomy, and the neurological outcome and quality of resection were better in awake craniotomy groups compared to general anesthesia groups with lesions in eloquent areas (18).
Some surgical factors preclude awake brain surgery, like the risk of massive bleeding in highly vascular tumors, uncomfortable surgical positions like prone or park bench for infratentorial tumors, or tumors where resection can cause severe pain, like those with dural involvement (19).
The preoperative assessment, beyond medical comorbidities and medications, such as antiepileptic medications and ongoing antiedematous therapy with dexamethasone, which should be continued until the surgery, also includes explaining the procedure, its reasons and its benefits to the patient. Beyond standard intraoperative monitoring, additional assessments are tailored to patient’s needs and may involve invasive blood pressure, central venous catheter, urinary catheter, BIS and entropy monitoring. Providing good analgesia on the scalp during an awake phase is facilitated by infiltrating local anesthesia on the surgical incision. Generally, two anesthetic techniques can be used for awake craniotomy: conscious sedation or monitored anesthesia care and the asleep-awake-asleep technique. Spontaneous ventilation is maintained through the procedure, with oxygen supplementation in patients with conscious sedation. Sedative drugs used for conscious sedation are midazolam, propofol (continuous infusion 50–150μg/kg/min), and dexmedetomidine (a bolus dose of 0.5–1μg/kg administered over 10 minutes, followed by a continuous infusion rate of 0.2–0.7μg/kg/h). Dexmedetomidine can be continued as an anxiolytic and analgesic in doses of 0.2μg/kg/h) during neurological testing. Analgesia is provided with fentanyl (boluses of 0.5–1μg/kg) and remifentanil (continuous infusion in doses from 0.01–0.05μg/kg/min) and continued in low doses during testing (0.01–0.02μg/kg/min) (2). Dexmedetomidine, a sedative and analgesic that acts as an α2-adrenoceptor agonist without causing respiratory depression, is commonly used for awake craniotomies (20). With conscious sedation as a technique and avoidance of manipulation of the airway, fluctuations of the hemodynamic and intracranial pressure are avoided. Anyway, with excessive sedation, there is a risk of airway obstruction, respiratory depression and apnea, all leading to hypoxia and hypercarbia.
The asleep-awake-asleep technique generally has an advantage over conscious sedation: It is more comfortable for the patient during painful parts of the procedure and reduces the risk of respiratory complications with controlled partial pressures of oxygen and carbon dioxide. Repeated airway manipulation during transitions from one phase to another can trigger laryngospasm or uncontrolled coughing, leading to increased intracranial pressure or intracranial bleeding. General anesthesia is induced with propofol, and ventilation usually is controlled by inserting a laryngeal mask or cuffed endotracheal tube. Anesthesia is maintained with continuous propofol, dexmedetomidine or volatile agent infusion. Analgesia is provided with boluses of fentanyl or continuous infusion of remifentanil. Propofol infusion is discontinued 15-20 minutes before neurological testing. Depending on the surgery, after the dural opening, the patient can be awakened for neurological testing and continuation of the surgery in the awake phase. Dexmedetomidine and remifentanil infusion may continue in low doses to ensure adequate analgesia and anxiolysis in alert but cooperative patients. When neurological testing and tumor surgery are completed, the patient is again induced into general anesthesia, usually with endotracheal intubation and mechanical ventilation. Techniques like video laryngoscopy and fiber-optic laryngoscopy are used for endotracheal reintubation.
Intraoperative seizures, respiratory adverse events, and loss of patient cooperation are the most common complications. Seizures occur in 2.2% to 21.5% of the patients, more often in younger patients with a history of seizures and receiving multiple antiepileptic medications. Frequently, there are seizures intraoperatively in low-grade glioma and frontal lobe tumor surgery (16). Ice-cold lactated Ringer (10-20mL) irrigation of the brain surface after opening the dura is usually an effective treatment (21) (22). Respiratory adverse events include airway obstruction, respiratory depression, apnea, hypoxia, hypercarbia and coughing. Hypoxia and hypercarbia potentially lead to surgical complications, increased intracranial pressure and open brain herniation. With adequate doses of sedative drugs, anesthesiologists should avoid oversedation and respiratory depression, and dexmedetomidine is advantageous for the lack of respiratory depression (23). The patient’s head should be adequately positioned for proper emergency reaction for airway access and ensuring ventilation. If complications occur and the patient is unresponsive or uncooperative because of an intracranial event, the anesthesiologist should secure the airway after preoxygenation and induce the patient in general anesthesia. Intracranial events, somnolence, hypoxia, hypercarbia, full bladder, hypotension, agitation, oversedation and inadequate analgesia, are causes of loss of patient cooperation.
Conclusion
The development of anesthetic techniques, including short-acting agents such as propofol, dexmedetomidine and remifentanil, has facilitated the performance of awake brain surgery. Careful planning and early recognition of intraoperative complications, if any, as well as ensuring proper oxygenation, ventilation, systemic and cerebral perfusion, and adequate analgesia during both the asleep and awake phases of the procedure, along with sedation for asleep patients, are the mainstays of anesthetic management for awake craniotomy. The maximal safe microsurgical resection of brain tumors can be achieved using this procedure, thereby minimizing damage to functional brain tissue in eloquent areas.
* Informed consent for the publication of the photograph with open eyes was obtain from the patient.
References:
- Piccioni F, Fanzio M. Management of anesthesia in awake craniotomy. Minerva Anestesiol. 2008;74(7- 8):393-408.
- P.H. Manninen, T.Y. Yeoh. Awake Craniotomy. Essentials of Neuroanesthesia. Elsevier. Inc. Academic Press. 2017, Pages 489-501. http://dx.doi.org/10.1016/B978-0-12-805299-0.00029-4.
- Dziedzic, T., & Bernstein, M. (2014). Awake craniotomy for brain tumor: indications, technique, and benefits. Expert Review of Neurotherapeutics, 14(12), 1405–1415. https://doi.org/10.1586/14737175.2014.979793.
- Capelle L, et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg. 2013;118(6):1157–1168.
- Lacroix M, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, the extent of resection, and survival. J Neurosurg. 2001;95(2):190–198.
- Sanai N, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8.
- Smith JS, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345.
- Singh K, Dua A. Anesthesia for Awake Craniotomy. [Updated 2023 Jul 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572053/.
- Han Q, Liang H, Cheng P, Yang H, Zhao P. Gross Total vs. Subtotal Resection on Survival Outcomes in Elderly Patients With High-Grade Glioma: A Systematic Review and Meta-Analysis. Front Oncol. 2020 Mar 18;10:151. doi: 10.3389/fonc.2020.00151. PMID: 32257941; PMCID: PMC7093492.
- Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, Patel AS, Rizk EB, Suki D, Sawaya R, Glantz M. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016 Nov 1;2(11):1460-1469. doi: 10.1001/jamaoncol.2016.1373. PMID: 27310651; PMCID: PMC6438173.
- Fiore G, Abete-Fornara G, Forgione A, Tariciotti L, Pluderi M, Borsa S, Bana C, Cogiamanian F, Vergari M, Conte V, Caroli M, Locatelli M, Bertani GA. Indication and eligibility of glioma patients for awake surgery: A scoping review by a multidisciplinary perspective. Front Oncol. 2022 Sep 21;12:951246. doi: 10.3389/fonc.2022.951246. PMID: 36212495; PMCID: PMC9532968.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005 Mar 10;352(10):987-96. doi: 10.1056/NEJMoa043330. PMID: 15758009.
- Gerritsen JKW, Zwarthoed RH, Kilgallon JL, Nawabi NL, Jessurun CAC, Versyck G, Pruijn KP, Fisher FL, Larivière E, Solie L, Mekary RA, Satoer DD, Schouten JW, Bos EM, Kloet A, Nandoe Tewarie R, Smith TR, Dirven CMF, De Vleeschouwer S, Broekman MLD, Vincent AJPE. Effect of awake craniotomy in glioblastoma in eloquent areas (GLIOMAP): a propensity score-matched analysis of an international, multicentre, cohort study. Lancet Oncol. 2022 Jun;23(6):802-817. doi: 10.1016/S1470-2045(22)00213-3. Epub 2022 May 12. PMID: 35569489.
- Ramakrishnan PK, Saeed F, Thomson S, Corns R, Mathew RK, Sivakumar G. Awake craniotomy for high-grade gliomas – a prospective cohort study in a UK tertiary-centre. Surgeon. 2024 Feb;22(1):e3-e12. doi: 10.1016/j.surge.2023.11.002. Epub 2023 Nov 25. PMID: 38008681.
- Garavaglia MM, Das S, Cusimano MD, Crescini C, Mazer CD, Hare GMT, Rigamonti A. Anesthetic approach to high-risk patients and pro-longed awake craniotomy using dexmedetomidine and scalp block. J Neurosurg Anesthesiol 2014; 26:226–33. (contr)
- Hervey-Jumper SL, Li J, Lau D, Molinaro AM, Perry DW, Meng L, Berger MS. Awake craniotomy to maximize glioma resection: methods and technical nuances over 27 years. J Neurosurg 2015; 123:325–39.
- Brown T, Shah AH, Bregy A, Shah NH, Thambuswamy M, Barbarite E, Fuhrman T, Komotar RJ. Awake craniotomy for brain tumor resection: the rule rather than the exception? J Neurosurg Anesthesiol. 2013 Jul;25(3):240-7. doi: 10.1097/ANA.0b013e318290c230. PMID: 23603885.
- Sacko O, Lauwers-Cances V, Brauge D, Sesay M, Brenner A, Roux FE. Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery. 2011 May;68(5):1192-8; discussion 1198-9. doi: 10.1227/NEU.0b013e31820c02a3. PMID: 21273923.
- See JJ, Lew TW, Kwek TK, Chin KJ, Wong MF, Liew QY, Lim SH, Ho HS, Chan Y, Loke GP, Yeo VS. Anaesthetic management of awake craniotomy for tumor resection. Ann Acad Med Singap. 2007 May;36(5):319-25. PMID: 17549277.
- Lechowicz-Głogowska B, Uryga A, Weiser A, Salomon-Tuchowska B, Burzyńska M, Fortuna W, Kasprowicz M, Tabakow P. Awake craniotomy with dexmedetomidine during resection of brain tumors located in eloquent regions. Anaesthesiol Intensive Ther. 2022;54(5):347-356. doi: 10.5114/ait.2022.123151. PMID: 36734444; PMCID: PMC10156559.
- Nossek E, Matot I, Shahar T, Barzilai O, Rapoport Y, Gonen T, Sela G, Grossman R, Korn A, Hayat D, Ram Z. Intraoperative seizures during awake craniotomy: incidence and consequences: analysis of 477 patients. Neurosurgery 2013; 73:135–40.
- Piccioni F, Fanzio M. Management of anesthesia in awake craniotomy. Minerva Anestesiol 2008; 74:393–408.
- Lapointe S, Perry A, Butowski NA. Primary brain tumors in adults. Lancet. 2018 Aug 4;392(10145):432-446. doi: 10.1016/S0140-6736(18)30990-5. Epub 2018 Jul 27. PMID: 30060998.