Trojikj T1,2, Kondeva Rogleva M1, Andonovska L1, Talevski D3, Kraleva S2, Stojceska V4
1 Faculty of Medical Sciences, University “Goce Delchev”, Shtip, Republic of North Macedonia
2Department of Anesthesiology and Intensive Care, City General Hospital “8th September”, Skopje, Republic of North Macedonia
3Department of Orthopedics and Traumatology, City General Hospital “8th September”, Skopje, Republic of North Macedonia
4Department of Otolaryngology, City General Hospital “8th September”, Skopje, Republic of North Macedonia
DOI: https://www.doi.org/10.55302/MJA2373097t
Abstract
For presentation of this case, we provided written consent from the patient.
69-years-old female patient came in hospital for operative treatment of total endoprosthesis of the right hip. She had multiple comorbidities regulated by chronic therapy. According to the pulmonary embolism severity index there was an increased risk for pulmonary thromboembolism (PTE). She received thromboprophylaxis Nadroparin calcium (Fraxiparine 0.6 ml sc) on the day of the hospitalization and it was regularly ordinated on daily basis. Perioperative and intra-operative, the patient was stable. Intraoperative, the patient was in OET anesthesia, in the course of 4 hours, we administered 3200ml of clear fluids and 350ml transfusions of deplasmatized erythrocytes. After extubating and leaving the operating room there was sudden drop in saturation 60-80%. She was transferred to intensive care, re-intubated, sedated, imaging methods were performed such as x-ray and CT angiography, where a diagnosis of pulmonary thromboembolism was established. Anticoagulant therapy was started immediately. There was an improvement in her condition, then she was extubated, conscious and oriented in space and time. After 2 days in intensive care unit she returned to the ward.
Key Words: fluids, general endotracheal anesthesia, pulmonary thromboembolism.
Introduction
Intraoperative acute pulmonary embolism is a serious complication in orthopedic patients and requires a risk assessment by anesthetists (1). Six hours fasting from food and 2 hours from liquids is generally recommended, and the patient should be encouraged to minimize the fasting period, thus avoiding dehydration (2). The most series of studies do not distinguish between emboli occurring during and after surgery, but Koessler et al. reviewed 4 series of patients undergoing total hip arthroplasty and found that the incidence of symptomatic intraoperative PE was between 0.6% and 10% (3). Cardiomegaly is the most common finding on chest radiograph in patients with PE. The anesthesiologists must be careful and monitor parameters that occur in this type of patients who are on mechanical ventilation, anesthetized. Hypotension and tachycardia are symptoms associated with PE (5).
Case Presentation
For presentation of this case, we provided written consent from the patient.
A 69-years-old female patient was presented to the Department of Orthopedics and Traumatology in our General Hospital as an elective admission for operative treatment of a total endoprosthesis of the right hip.
While doing the anamneses, we came to valuable information that the patient has been operated three times. Arthrosis changes appeared for the first time in 2005 when an endoprosthesis was implanted on the left hip. Due to the progression of degenerative changes, the right hip was operated, and arthroplasty was done in 2015. In 2020, due to an infection at the site of the implanted prosthesis, it was removed.
After clinical, paraclinical and radiological analyses, an indication for operative treatment was established. The patient had several comorbidities – hypertension, diabetes type 2 and dyslipidemia, extremely obese type 3 (BMI 46.5), chronic renal failure stage 4 (without dialysis treatment). Accordingly, she received a beta blocker, angiotensin II receptor blockers (ARB), statin and insulin subcutaneously. Hemostasis and d-dimers were tested regularly from the first day of her hospitalization and they were in reference value. She didn’t consume alcohol, was not addicted to cigarettes and other drugs. An acetabular defect of the right acetabulum was determined according to Paprosky classification – grade I. The type and risks from the operation and anesthesia were explained to the patient and the family. There was high risk of thromboembolism and Nadroparin calcium (Fraxiparine 0,6ml) was ordinate. The operative treatment started on 21.01.2022 in OET anesthesia. The patient was placed in a supine position in the operating theatre. We did latero-anterior approach in layers and en route hemostasis, then the right hip was approached. The acetabulum was processed, an acetabular component number 49mm (All-Poly Acetabular Cup- Zimmer) was applied. Then the femoral canal was processed, and femoral stem No. 7.5 Original M.E. was implanted. Muller-Zimmer, a +3.5mm/28mm head (VerSys-Zimmer) was placed on the stem. The operative wound was washed, repositioned, closed in layers, dressed, and bandaged. We implanted total cemented endoprosthesis.
During the induction we gave Midazolam 2mg, fentanyl 0.05mg, propofol 160mg, 2% lidocaine 40mg, rocuronium 50mg. During the intervention we had two syringe pumps for our total intravenous anesthesia – TIVA, propofol 400mg/50ml/5-10ml/h, remifentanil 2mg/40ml/5-10ml/h. Fentanyl was added according to vital signs as well as a non-depolarizing myorelaxant. The patient received 1700ml NaCl 0.9% Physiological Saline, 1000ml Ringer’s, 500ml plasma expander HES 6%, 350ml transfusions of deplasmatized erythrocytes. The operation lasted 4 hours. During the operative period the patient was stable. On Figure 1 we can see the values of blood pressure and heart rate intraoperatively. Antibiotic, analgetic, gastroprotective, antiemetic therapy was given.
The saturation during surgery ranged from 98%-100%, and the capnograph had values within normal limits.
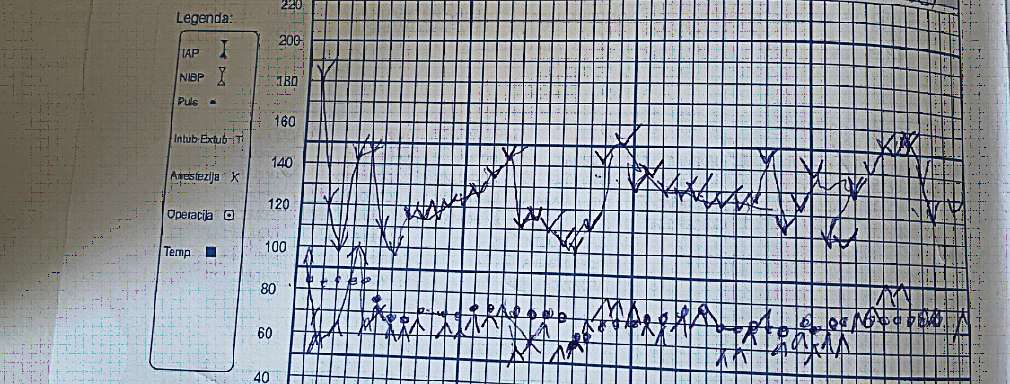
Figure 1. Systolic pressure is noted with the port facing up, diastolic pressure is noted with the port facing down, pulse is noted with a dot. One square indicates a 5-minute interval.
Table 1. Drug dose and hour during surgery when given.
| Drugs given during intervention/ timeline | 0-1 hour | 1-2 hour | 2-3 hour | 3-4 hour |
| Fluids | 1200ml (NaCl 0.9%) | 1000ml (Ringer),
500ml 6% HES |
500ml (NaCl 0.9%) | |
| Midazolam | 2mg | |||
| Lidocaine 2% | 40mg | |||
| Fentanyl | 0.1mg | 0.25mg | 0.1mg | |
| Rocuronium | 70mg | 40mg | 10mg | |
| Propofol | ||||
| Remifentanil | ||||
| Tranexamic acid | 1,0gr/i.v. | |||
| Paracetamol | 1,0gr/i.v. | |||
| Ketoprofen | 160mg/i.v. | |||
| Tramadol | 100mg/i.m | |||
| Clindamycin | 600mg/i.v. | |||
| Cefriaxone | 2gr//i.v. | |||
| Famotidine | 20mg/i.v. | |||
| Metoclopamide | 10mg/i.v. | |||
| Ephedrine | 6mg/i.v. | |||
| Furosemide | 10mg/i.v. | |||
| Methylprednisolone | 80mg/i.v. | |||
| Blood transfusion | 350ml RBE (red blood erythrocytes) | |||
| Neostigmine | 2.5mg | |||
| Atropine | 1.0mg |
When the operation was done, the patient was awakened, extubated, and placed into the ward. After a short period of time, the patient developed breathing-dyspnea with cyanotic skin and mucous membranes. The patient was awake, on auscultation there were decreased breath sounds. Saturation – Sp02-59-83% was with oxygen mask. Immediately we gave methylprednisolone 120mg, dexamethasone 4mg, aminophylline 250mg, furosemide 20mg, and another dose of nadroparin calcium (Fraxiparine) 0.6mg s.c. After the given therapy, the condition did not improve. Due to the drop in saturation and no improvement in her overall condition and symptoms, the patient was transported to the intensive care. We intubated her, after which she was placed on mechanical ventilation and her saturation improved significantly to 99%. Propofol sedation was started. At that time, we suspected that patient might be developing pulmonary embolism. A chest X-ray was taken, where reduced transparency was noted on the left side of the lung in the middle and basal parts, as well as an increased cardiac shadow. Cardiomegaly is a very common X-ray sign in patients with pulmonary embolism ( PE) (5). After the X-ray image, a CT angiography was performed, defect in the filling of the pulmonary artery of the lower lobe in the left lung was noted, truncus pulmonalis and other pulmonary arteries were in the reference value.
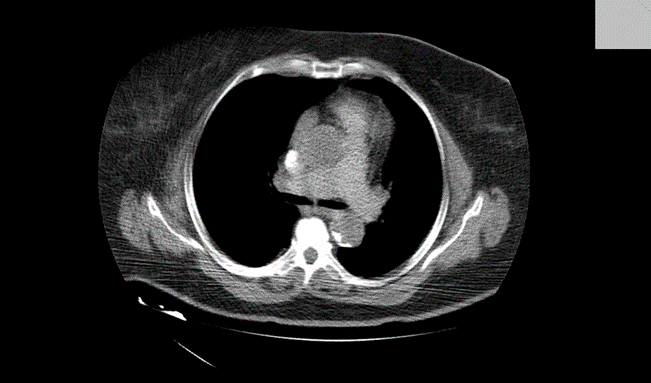
Figure 1. Thromboembolism shown on CT with contrast.
We discussed whether we should include thrombolytic therapy with streptokinase drugs. Because of the fear of bleeding, we decided to continue with factor Xa inhibitors – Nadroparin calcium. Hemostasis was done and d-dimers were 9000ngr/ml. In consultation with doctor specialist – transfusiologist, Amp.Nadroparin calcium (Fraxiparine) 2×0.6ml, or 5700 I.E. were prescribed s.c. The next day, 12 hours after intubation sedation was turned off. The patient was awake, on auscultation vesicular breathing, slightly weakened on left basal part of left lung. We placed her on continuous positive airway pressure-CPAP Fi02=0.5; PEEP=0.5 Sp02-99%. Her condition was improving hour by hour and we transported her to the ward. Blood tests were done every day, on the first day post-operatively, there was reduction of blood elements and reactive leukocytosis as a result of anemia. We substituted blood through transfusion of erythrocytes, and the condition was improving. The drain was removed on the first postoperative day. The operative wound was without signs of inflammation and infection. Other inflammatory parameters such as sedimentation were normal, C-reactive protein was slightly elevated. Therefore, a swab from the wound was taken. It was negative for pathogenic aerobic and anaerobic bacteria and fungi were not detected. Physical medicine was performed during the entire hospital stay, from the second day post-operatively was in vertical position, good condition and instruction for home were given. At home, she would need to regulate her blood sugar – glycemia, to receive her chronic therapy, as well as to continue with Amp.Nadroparine calcium (Fraxiparine) 0.8ml -7600 I.E. s.c. for 14 days and Tbl.Rivaroxaban 15mg 2×1. To continue with regular blood checks for hemostasis and d-dimers and according to the results to continue therapy.
Diagnosis
Angiography is considered the gold standard for the diagnosis of PE. It is an expensive method, not available in all hospitals and not without risk. According to Stein et al., there is a 4% incidence of serious complications (including death and renal failure) in intensive care units in patients who underwent CT angiography (4).
Discussion
Our patient had several risk factors: hypertension, diabetes, obesity type 3, serious surgery – hip arthroplasty. A prospective study by Goldhaber et al. showed that obesity (BMI>29kg/m2) and cigarette smoking (N>35 cigarettes/day) are independent risks factors for PE in women, with a relative risk of 2.9 and 3.3, respectively (7). PE may be viewed as part of the cardiovascular disease continuum and common risk factors – such as surgery, cigarette smoking, obesity, hypercholesterolemia, hypertension and diabetes mellitus. Common ECG findings associated with PE are ST segment and T wave abnormalities. Non-specific ST changes, ST elevation and depression, or T wave inversion, are found in approximately 50% of patients. Complete right bundle branch block and T wave inversions in the precordial leads are the findings that correlate best with severity of PE. The diagnosis is established with the help of CT angiography, and aggressive treatment should start immediately with low molecular weight heparin. Systemic therapy for thromboembolism includes thrombolytic therapy with streptokinase drugs. Postoperative, thrombolytic therapy can lead to increased bleeding, so anticoagulant therapy (Enoxaparin or fraxiparine 1.0mg/kg every 12 hours or 1.5mg/kg once a day) can be therapy of a choice. Reperfusion is usually established with this therapy at the site of TE, the incidence of hemodynamic instability decreases. Collapse of the patient and increased risk of hemorrhagic brain infarction and serious non- brain hemorrhage (8) can occur. Hemodynamic instability is a major and significant risk factor before, intra and in postoperative period in PE (9).
Conclusion
Patients from risk groups should be evaluated before operation and properly treated. Time management is one of the key factors in the patient’s overall well-being and survival. Experienced physician who is good in teamwork is important. In differential diagnosis it should be considered.
References
- Smith I, Kranke P, Murat I, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiology. 2011.
- Koessler MJ, Fabiani R, Hamer H, Pitto RP. The clinical relevance of embolic events detected by TEE during cemented total hip arthroplasty: a randomized clinical trial. Anesth Analg 2001.
- Capan LM, Miller SM. Monitoring for suspected pulmonary embolism. Anesthesiology Clinic North America 2001.
- Stein PD, Athanasoulis C, Alavi A, et al. Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation 1992.
- Еliott CG, Goldhaber SZ, Visani L, DeRosa M. Chest radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry. Chest 2000.
- 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. European Heart Journal 2014.
- Goldhaber SZ, Grodstein F, Stampfer MJ, et al. A prospective study of risk factors for pulmonary embolism in women. JAMA 1997.
- Aiping Y, Shuangyin Z, Yanhong X, Rongzhi Z. Management of intraoperative acute pulmonary embolism in a patient with subarachnoid haemorrhage undergoing femoral fracture repair. Journal of International Medical Research 2019.
- Timothy E.М, Paul S.M. Perioperative Fluid Therapy for Major Surgery. Anesthesiology 2019.