UDK: 616-089.5:618.3
Eftimova B1
1Faculty of Medical Sciences – Department of Anesthesiology with Emergency Medicine and Intensive Care, “GoceDelchev” University – Shtip
Introduction
It has been estimated that each year between 0.5-2.2% of pregnant women receive anesthesia for various surgical interventions of a non-obstetric nature during their pregnancy. The purpose of the operative intervention may be:1.to prolong the pregnancy, 2. it is not related to the pregnancy or 3.to correct the anomalies of the fetus. Therefore, understanding the effects of various anesthetic drugs and techniques on the mother and fetus is essential for safe anesthesia in pregnant women undergoing operative intervention. The operation can be indicated at any stage of pregnancy. The anesthesiologist must provide safe anesthesia for both,the mother and the fetus. For the mother, safety is related to the physiological adaptations associated with pregnancy, which require adjustments to standard anesthesia techniques. Fetal safety refers to the teratogenicity of certain drugs and anesthetics, avoiding fetal asphyxia, and avoiding premature labor and delivery.
Maternal safety: Maternal Physiological Adaptations to Pregnancy
A pregnant woman undergoes significant physiological adjustments during pregnancy. Most of these changes are due to the mechanical effects of an enlarged uterus, hormonal changes associated with pregnancy, increased metabolic demands and a low-resistance placental circulation. Minute ventilation and oxygen consumption increase, while residual volume and functional residual capacity decrease. Therefore, oxygen reserves decrease, and pregnant women develop hypoxia and hypercapnia with hypoventilation or apnea more quickly. Airway management by face mask, laryngeal mask, or endotracheal intubation can be technically difficult in pregnant women due to increased anteroposterior chest wall diameter, breast enlargement, laryngeal edema and weight gained affecting the soft tissues of the neck.
Cardiovascular changes – Cardiac output increases gradually starting from the 8th week of gestation and reaching its maximum increase by the end of the 2nd trimester. Both heart rate and stroke volume are increased, resulting in a 50% increase in cardiac output by the end of the second trimester. Myocardial contractility remains unchanged, but systemic vascular resistance is reduced. This is primarily due to progesterone, as well as the presence of low placental resistance. From about mid-pregnancy, women in the supine position are at risk for aortic and vena cava compression by the gravid uterus. Physiological compensation for aortocaval compression may be compromised by anesthetic techniques (spinal, epidural, or general) that prevent the sympathetic nervous system from responding adequately and may result in profound hypotension. Therefore, it is important to avoid this by placing the patient in the left lateral position.
Gastrointestinal Changes – Due to mechanical and hormonal changes, pregnant women are at increased risk for gastric acid aspiration during induction of anesthesia or sedation. Gastroesophageal sphincter tone is reduced, and although gastric motility remains normal during pregnancy, it is significantly impaired by opioid administration, onset of labor, pain, trauma and more. For pregnant women in the second or third trimester, or those with a history of reflux esophagitis, it is necessary to use the so-called “full stomach” techniques.
Fetal Safety- Avoidance of Fetal Asphyxia
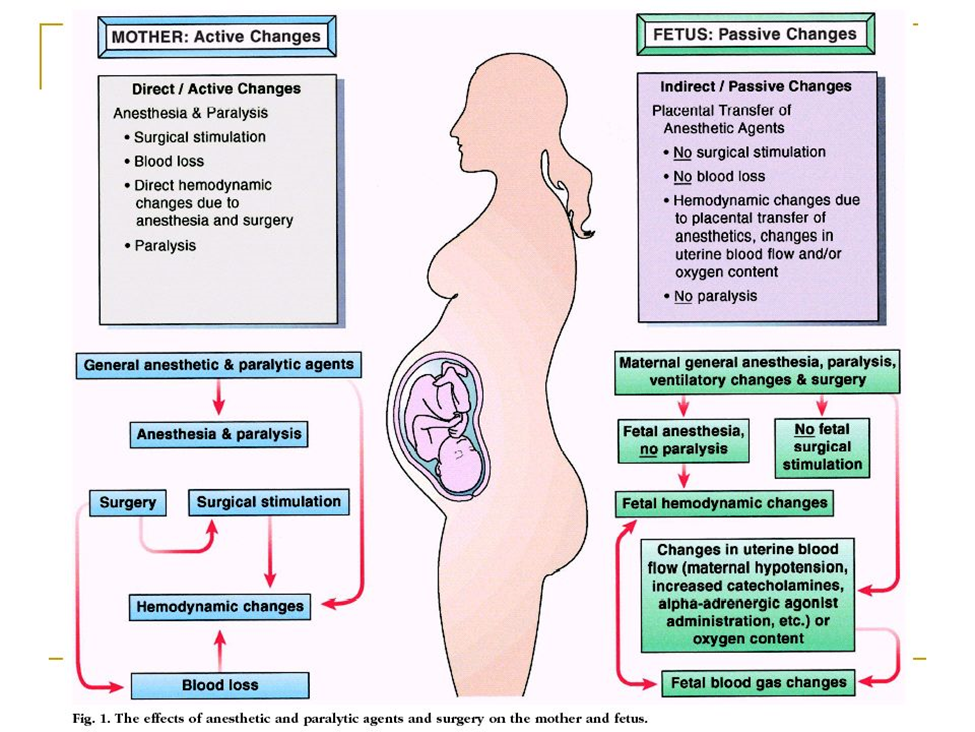
During pregnancy, the most important and serious risk for the fetus from the mother’s surgery is intrauterine asphyxia. The greatest challenge for the anesthesiologist is, therefore, to avoid fetal asphyxia by maintaining normal maternal oxygenation and hemodynamics. Maternal oxygenation, carbon dioxide levels, blood pressure and uterine tone are factors that should be controlled during surgery to avoid fetal asphyxia. It is extremely important to avoid hypoxia, hypercarbia, hypocarbia, maternal hypotension and uterine hypertonus during non-obstetric surgery. This is probably much more important than concerns about the teratogenicity of various anesthetic drugs. Mild periods of hypoxemia of short duration are well tolerated. However, prolonged, or severe maternal hypoxemia causes uteroplacental vasoconstriction and decreased uteroplacental perfusion, resulting in fetal hypoxemia, acidosis and ultimately fetal death. Hyperoxia is not dangerous, contrary to what was previously thought. It has been clearly demonstrated that hyperoxia does not result in increased uterine vascular resistance, nor it reduces fetal oxygenation as measured by fetal scalp gas analysis. Maternal hypercarbia directly induces fetal respiratory acidosis. Severe fetal respiratory acidosis causes fetal myocardial depression. Hypercapnia also results in vasoconstriction of uterine arteries and decreased uterine blood flow. Similarly, hypocapnia also results in decreased uterine blood flow and ultimately fetal acidosis. Vasoactive drugs that reduce uterine blood flow, such as α -adrenergic agents, dopamine or epinephrine, are not ideal agents for treating maternal hypotension; although blood pressure may increase, blood flow to the uterus may remain decreased. For the treatment of maternal hypotension, ephedrine has long been considered the first choice. However, recent data suggest that phenylephrine is equally effective in maintaining normal maternal blood pressure and that phenylephrine produces significantly better fetal acid-base balance, at least in term pregnancies undergoing caesarean section under regional anesthesia. Therefore, it is now considered preferable to treat maternal hypotension with phenylephrine. Several drugs commonly used in anesthesia, such as ketamine or IV local anesthetics, may cause uterine hyperactivity and should be avoided. Maternal oxygen administration will increase fetal oxygenation, however, the fetus is never at risk of hyperoxia, as fetal oxygen tension will not exceed approximately 65mmHg, even with maternal administration of 100% oxygen.
Teratogenicity of Anesthetic Drugs
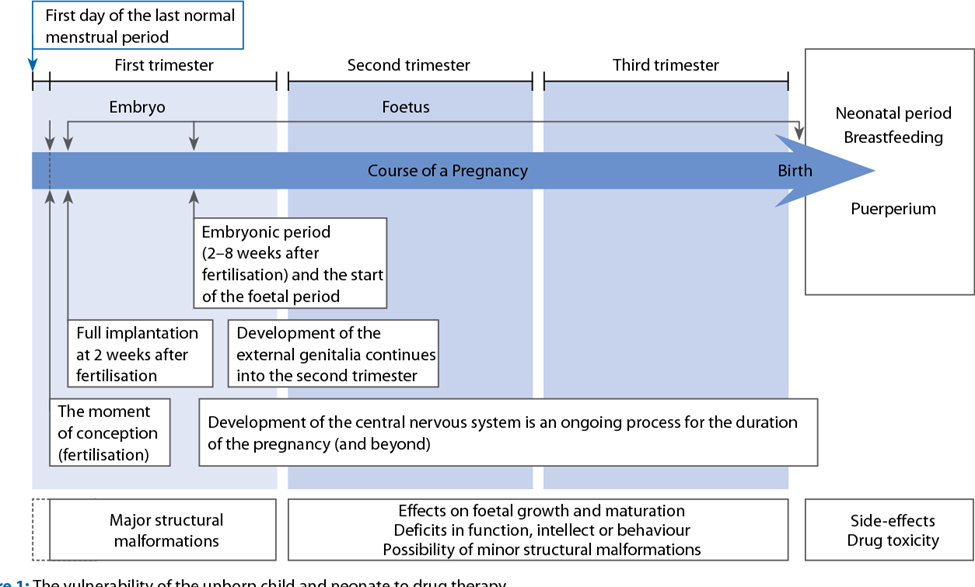
Medicines can be toxic to the developing embryo and fetus. The first trimester of pregnancy, i.e. the stage of embryonic development and organogenesis is of particular importance because the teratogenic effects of certain drugs affect the normal development of the unborn child at a structural or functional level. A teratogen is a drug or other chemical substance that can affect the normal development of the embryo and cause recognizable congenital defects.
Anesthetic drugs affect intra- and intercellular exchange and have known effects on cell mitosis and DNA synthesis. Therefore, all anesthetic agents can be potentially teratogenic. The teratogenicity of the drug is determined by the dose administered, the method of administration, the time of exposure of the fetus and the species that is exposed to the drug. The time of exposure to the anesthetic is critical. During the first 15 days of pregnancy, an all-or-nothing phenomenon occurs: the fetus is lost, or the fetus is preserved completely intact. Structural abnormalities may occur during organogenesis (15-56 days). After this period, functional changes may be observed, but structural abnormalities are rare. Because prospective clinical studies are impractical, unethical, and would require large numbers, most of our knowledge comes from animal studies, accidental exposures, and reports from series of patients who underwent anesthesia while pregnant. Although most of the anesthetics are known as teratogens in certain species, when administered at a sufficiently high dose or when administered directly to the fetus, most of the agents are, however, perfectly safe in the clinical setting. We now know that local anesthetics, volatile anesthetics, induction drugs, muscle relaxants and opioids are not teratogenic when used at clinical concentrations, and when normal maternal physiology is maintained. It is probably best to avoid nitrous oxide during pregnancy, as it is not necessary to use this agent to provide safe and effective anesthesia. Nitrous oxide has known effects on DNA synthesis and has been shown to have teratogenic effects in animals. Nitrous oxide has been shown to inactivate methionine synthetase, which in turn inhibits thymidine and DNA synthesis, inhibits cell division, and potentially disrupts other biochemical pathways. In methylation reactions,to date, there are no clinical data linking these cellular actions to teratogenic outcomes. Laboratory studies of the teratogenicity of inhalation agents in rodents indicate that modern volatile anesthetics at trace and subanesthetic concentrations do not result in adverse reproductive or teratogenic effects. Nitrous oxide is a weak teratogen in rodents when given for long periods. However, coadministration of isoflurane reverses the fetal lethality and teratogenic effects of nitrous oxide without affecting methionine synthetase activity. Large survey studies that have taken into account the results of women who have undergone surgery during pregnancy, do not indicate an increase in congenital anomalies in their offspring, but an increase in the risk of abortions, growth restriction and an increased frequency of small and very low birth weight babies. Birth weight for reasons attributable to the surgery itself, but not to anesthesia. Some smaller retrospective studies have suggested an association with neural tube defects in the first trimester exposure to anesthesia. However, the patient’s primary illness, the site of the operation, or the surgical procedure are more likely to increase the risk of abortion than exposure to anesthesia. Although many pregnant women undergo anesthesia and many more are exposed through the anesthetic profession each year, the teratogenic risk of anesthetic agents in humans must be assessed based on incomplete data. Available studies suggest, for a surgical procedure, that administration of nitrous oxide or volatile, opioid, regional, or local anesthetics to pregnant women will have no deleterious effects on embryonic or fetal development and no clinical significance for adverse neonatal outcome. The danger of teratogenic effects from currently available anesthetic or sedative drugs remains only a potential risk. No anesthetic, opioid analgesic, sedative-hypnotic, or anxiolytic appears to be teratogenic or safer than another agent. Long-standing relative contraindications and concerns about the use of benzodiazepines, especially in the first trimester, were later dismissed. And they are used as preoperative medications for women to treat pain or anxiety, because catecholamines that are increased by pain or anxiety can negatively affect blood flow to the uterus as well.
Prevention of Premature Birth – Fetal Monitoring
After surgery performed during pregnancy, the risk of premature birth or abortion is increased, especially if the surgery involves intra-abdominal procedures. Prophylactic tocolytic therapy is controversial, as tocolytic agents have significant side effects and maternal efficacy during non-obstetric surgery has not been proven. Tocographic monitoring during the first hours or days postoperatively is recommended to detect and treat preterm labor as early as possible. Nowadays, it is recommended to routinely use fetal heart rate (FHR) monitoring when feasible. Surgery and anesthesia can affect uterine activity and placental perfusion, and thus fetal oxygenation and fetal heart rate. Fetal heart rate can also be directly affected by drugs that readily cross the placenta, or indirectly by their effect on maternal hemodynamics. Monitoring of the fetus and uterus during surgery is often possible, but in some circumstances access may be difficult. However, such monitoring may be impractical in emergency situations, has not been documented to improve fetal outcome, and requires expertise often not possessed by regular staff. Misinterpretations can lead to unsafe interventions. When used, appropriate personnel trained in basic fetal heart rate interpretation should be immediately available. Unfortunately, there is no evidence to show that the use of intraoperative FHR monitoring improves fetal outcome. The issue remains controversial, but many obstetric textbooks advise monitoring whenever feasible.
Laparoscopy
According to many authors there is a concern for the well-being of the fetus during laparoscopy primarily due to direct trauma to the uterus or fetus and fetal acidosis from absorbed carbon dioxide (CO2). Also, due to increased intra-abdominal pressure, maternal cardiac output, the utero-placental perfusion can be reduced. Animal data have confirmed these suspicions. However, clinical experience, using careful surgical and anesthetic technique is favorable. Reedy et al. compared laparotomy and laparoscopy performed in pregnancy in over 2 million pregnancies in Sweden over a 20-years period. These authors included 2,181 laparoscopies and 1,522 laparotomies with a gestational age between 4 and 20 weeks. They compared 5 fetal parameters (birth weight, gestational duration, growth restriction, infant survival and fetal malformations) for each type of surgery with the overall outcome in the non-operated population. Premature delivery, growth restriction and low birth weight were more common in the operated group compared to the general population. No differences were identified between laparoscopy and laparotomy. Thus, the following guidelines issued by the American Society of Gastrointestinal Endoscopic Surgeons regarding laparoscopic surgery during pregnancy, suggest that whenever possible, surgery should be postponed until the second trimester. Preoperative obstetric consultation should always be obtained.
Other Important Changes
As a result of increased plasma volume, anemia occurs, despite the increase in red blood cell volume. Pregnancy is also associated with benign leukocytosis, causing a hypercoagulable state with an increase in the most of coagulation factors, as well as coagulation and fibrinolysis. Thus, pregnancy is a state of compensated intravascular coagulation. Thrombocytopenia can occur in up to 1% of pregnancies without signaling preeclampsia. The hypercoagulable state puts the pregnant patient at high risk for postoperative thromboembolic complications. The glomerular filtration rate increases by 50% during pregnancy and as a result, creatinine clearance is increased by 50%. Serum creatinine concentrations, therefore, are reduced by almost 1/3. Anesthetic requirements are significantly reduced for both inhaled and intravenous anesthetic agents. Pregnancy is associated with increased sensitivity to inhaled anesthetics with minimal decreases in alveolar concentration of up to 40% reported. Similarly, sensitivity to intravenous agents is also increased. A lower amount of anesthetics given during spinal and epidural anesthesia is required to produce similar dermatomal spread in pregnancy compared to non-pregnant patients. This is due to the hormonal, as well as mechanical effects of the enlarged uterus. Nondepolarizing muscle relaxants have a prolonged duration of action, whereas the duration of action of succinylcholine is not affected by pregnancy.
Practical Approach
These physiological changes in pregnant women require anesthesiologists to adapt their routine anesthetic technique. Ideally, the surgery should be performed during the second trimester. Laparoscopy is possible. Acid aspiration prophylaxis (a combination of H2-blocker, oral sodium citrate 30 mL and metoclopramide) is recommended to reduce gastric contents and increase gastric pH. This clearly results in reduced morbidity and mortality when accidental aspiration occurs. Adequate left lateral tilt positioning of the pregnant woman in supine position (at least 20° left lateral tilt) is required to avoid recumbent hypotensive syndrome. This should be done from the 2nd trimester onwards. The pregnant patient is more prone to hypoxia due to reduced functional residual capacity and increased oxygen consumption. Therefore, careful denitrogenation is recommended before induction of general anesthesia. Rapid sequential induction should be performed using cricoid pressure and a fast-acting muscle relaxant. The drug of choice remains succinylcholine. Rocuronium would be an alternative. However, it has a significantly prolonged duration of action that is difficult to detect during artificial ventilation. All anesthetic agents can be used. The volatile agent is useful in preventing premature uterine activity. It is prudent to avoid nitric oxide. Hypoxemia, hypercarbia and hypocarbia should be avoided, and hypotension should be treated aggressively with intravenous fluids and phenylephrine or ephedrine. Good postoperative analgesia must be provided. Pregnant patients are more prone to thromboembolic complications and appropriate prophylactic measures should be taken, including prophylactic administration of low molecular weight heparins.
The Effect of Anesthetics on the Fetus
Teratogenic studies of various anesthetic agents have been studied mainly in animals. It is very difficult, but also impractical to mirror these results to humans. Fortunately, there are no commonly used anesthetics that are known teratogens when given acutely.
Sedative and Hypnotic Agents
Barbiturates have been used in humans for induction for many years. Although there are conflicting animal reports regarding the teratogenic effect of barbiturates, these agents are safe in pregnant women. Phenothiazineshave also been reported to have no adverse effect for anesthesia for non-obstetric surgery. The association of minor sedatives with teratogenicity is controversial, although retrospective studies have shown diazepam and chlordiazepoxide to be associated with congenital malformations. On the other hand, more recent studies have not found an increased risk of congenital anomalies after diazepam use. No teratogenicity has been observed for midazolam. Recently published literature on women who have attempted suicide during pregnancy by taking large doses of drugs such as diazepam, medazepam, promethazine and meprobamate, has not shown that these drugs are fetotoxic.
Opioids
Geber and Schramm observed the teratogenicity of a wide variety of narcotics administered to pregnant hamsters during critical periods of fetal central nervous system development. Comparative studies using single or multiple doses have shown increased fetal anomalies with diacetylmorphine, thebaine, pentazocine, morphine, hydromorphone as well, such as meperidine. On the other hand, other authors noted that chronic administration of morphine, fentanyl, sufentanil or alfentanil in pregnant rats was not associated with any teratogenic effect. There is also no evidence that these opioids are associated with teratogenicity in humans.
Muscle Relaxants
There is no evidence of a negative effect on the development of the fetus after the use of muscle relaxants.
Local Anesthetics
In a very large study conducted by the Collaborative Perinatal Project and in other studies, no evidence of teratogenicity was found in pregnant rats following administration of benzocaine, procaine, tetracaine, or lidocaine. In contrast, cocaine use has been associated with birth defects in both humans and animals. This could be explained by cocaine-induced vasoconstriction and, hence, fetal tissue hypoxia.
Oxygen and Carbon Dioxide
Hypoxia, as well as hypercarbia, has been associated with teratogenicity in animal species.
Inhalation Anesthetics
Nitrous Oxide
Interest in the teratogenic effect of nitrous oxide increased significantly among anesthesiologists after Nunn and colleagues observed the effect of short-term administration of a nitrous oxide anesthetic on plasma concentrations of methionine, tryptophan, phenylalanine, and S-adenosylmethionine in humans. Using nitrous oxide intraoperatively and up to 24 hours postoperatively, Scatzel and colleagues observed a significant decrease in plasma methionine concentration after major vascular surgery in humans and recovery that occurred only after cessation of nitrous oxide administration. The main reason for the reduced concentration of methionine in the plasma is related to the inhibition of the enzyme methionine synthetase. Thus, the teratogenic effect of nitric oxide may be related to interference with DNA synthesis by altering folate metabolism. Keeling and his colleagues observed the effect of pretreatment with folic acid on the teratogenic effect of nitrous oxide in rats such that skeletal abnormalities in the group receiving nitrous oxide without pretreatment were five times greater compared to the control group, which had been pretreated with folic acid, where the changes were insignificant. Hence, the authors concluded that the mechanism of teratogenicity after exposure to nitrous oxide may not be related to interference with DNA synthesis, but to a physiological effect of nitrous oxide in reducing uterine blood flow due to increased sympathetic activity. In summary, although there is a relationship between nitrous oxide use and teratogenicity in rats, the exact mechanism is currently unclear. In humans, brief exposure to nitrous oxide during the second trimester was not associated with any adverse effect.
Halogenated Anesthetics
Halothane, enflurane, and isoflurane at physiological minimum alveolar concentrations have not been associated with any teratogenicity in rats, nor has evidence of teratogenicity in humans been observed with these agents. The newer inhaled agents desflurane and sevoflurane are also not associated with any teratogenicity.
Effects on the Fetal Brain: Behavioral Teratogenicity
As with all non-human animal studies, it is difficult to extrapolate the degree of risk from anesthetics to humans undergoing general anesthesia or fetuses exposed in utero to anesthesia due to maternal surgery. Hopefully, in the future, a better understanding of the mechanism of toxicity will also point to strategies to block the harmful effects. While laboratory and eventual clinical trials continue, it is reasonable to assume that general anesthetics are potentially toxic to the developing fetal brain, and their use in obstetric anesthesia should continue to be reserved for emergencies only.
Recommendations to Minimize Abortion or Premature Birth
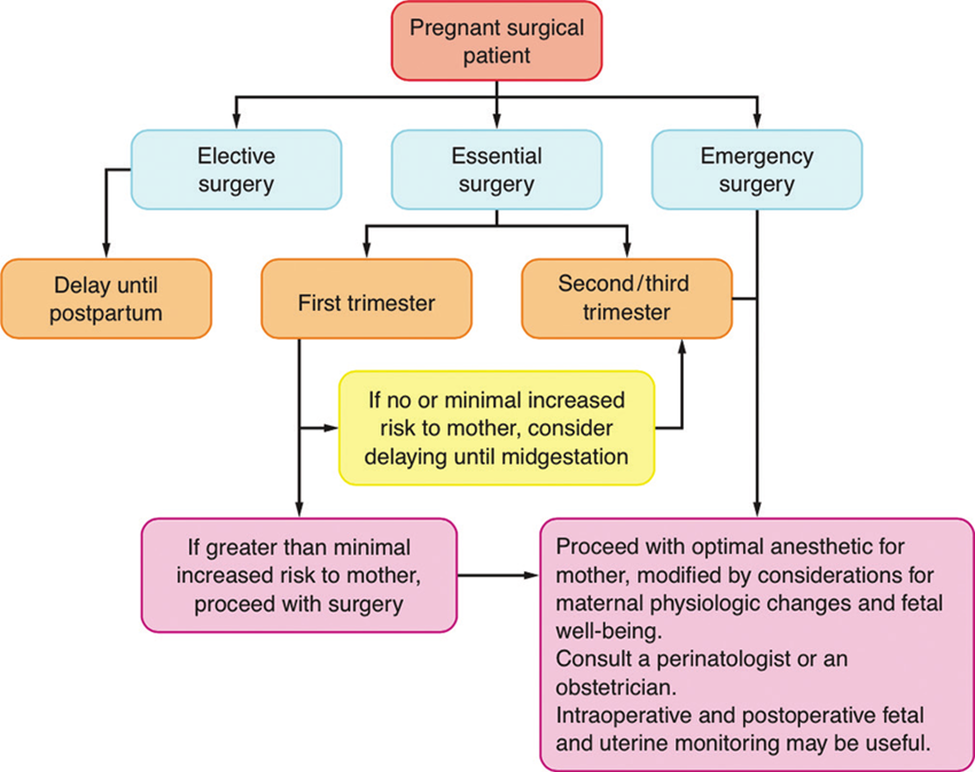
If an operative intervention is planned, it should be postponed until after delivery. In semi-urgent cases, it is best to postpone the operation until after the first trimester. In emergency cases, the anesthetic chosen should depend on the site and extent of the operation to be performed. If possible, regional anesthesia, spinal, epidural, or caudal block is recommended. However, if necessary, general anesthesia can be applied. Benzodiazepines and opioids can be given preoperatively for premedication, if necessary. A routine antacid should be used before induction, and rapid sequence induction is often chosen. Although there is no general consensus, it is reasonable to use endotracheal intubation for longer or more extensive procedures. Then depolarizing or non-depolarizing muscle relaxants. Anesthesia can be maintained with nitrous oxide, oxygen and halogen anesthetics. Morphine, fentanyl, sufentanil, or alfentanilmay be used as analgesics. Hyperventilation should always be avoided as it may decrease uteroplacental perfusion, as well as shift the maternal hemoglobin dissociation curve to the left. For regional anesthesia, maintenance of normal blood pressure is absolutely necessary, and routine use of face mask oxygen is recommended. Regardless of whether general or regional anesthesia is chosen, moving to the left lateral position of the operating chair when lying on the back is mandatory from the middle of the second trimester onward. Routine monitoring should include blood pressure, electrocardiogram, oxygen saturation, capnography and temperature. Close communication between the anesthesiologist and obstetrician regarding fetal heart rate monitoring and interpretation of results is essential. Because most of the drugs used for general anesthesia can alter the fetal heart rate, the baseline fetal heart rate should be the main indicator of fetal well-being during general anesthesia. Depending on the location of the surgery, tocodynamometrymay be used to monitor uterine contractions. This is already becoming routine in the postoperative period, when treating pregnant women with premature contractions with tocolytics. Nowadays, laparoscopic surgery during pregnancy is successfully used, but one must have basic knowledge about the physiological changes during pregnancy. During laparoscopic cholecystectomy, women are placed in a head-down position, so these positions can have significant cardiovascular and respiratory effects. Peritoneal insufflation pressure should be kept low because of the possibility of aortocaval compression. Ventilation should be optimal to maintain end-tidal PCO2 at 32-34mmHg. Intra-abdominal pressures were maintained around 15mmHg. Adjusting ventilation to maintain ETCO2 also maintains optimal maternal arterial CO2. Also, cardiac output decreases by about 30% during laparoscopic surgery in pregnant women, and therefore vasopressors (ephedrine) should be given to maintain blood pressure within 20% of baseline.
Conclusion
Elective procedures should be delayed until approximately 6 weeks postpartum, when the physiologic changes of pregnancy have passed, and fetal well-being is no longer a concern. Women of reproductive age should be asked about their last menstrual period, informed of the potential risks, and offered pregnancy testing if their menstrual history is uncertain, or they seek to avoid planned procedures during early pregnancy. Despite the lack of clinical evidence, delaying surgery until the second trimester, when possible, may reduce the risks of teratogenicity and miscarriage. Whenever major surgery is undertaken on a pregnant patient, a perinatologist or obstetrician should be consulted to assist in perioperative management, to diagnose and properly manage possible preterm labor, and to try to avoid preterm labor. Informing the obstetrician or perinatologist about any surgical procedure may be in the patient’s best interest.
References:
- Kitson K, Ormond K, Pergament E. Surgery in Pregnancy; 2000.
- Gidai J, Acs N, Banhidy F, Czeizel AE. No association found between use of very large doses of diazepam by 112 pregnant women for a suicide attempt and congenital abnormalities in their offspring. ToxicolInd Health. 2008;24(1–2):29–39.
- Petik D, Acs N, Banhidy F, Czeizel AE. A study of the potential teratogenic effect of large doses of promethazine used for a suicide attempt by 32 pregnant women. ToxicolInd Health. 2008;24 (1–2):87–96.
- Timmermann G, Acs N, Banhidy F, Czeizel AE. A study of teratogenic and fetotoxic effects of large doses of meprobamate used for a suicide attempt by 42 pregnant women. ToxicolInd Health. 2008;24(1–2):97–107.
- O’Leary G, Bacon CL, Odumeru O, et al. Antiproliferative actions of inhalational anesthetics: comparisons to the valproate teratogen. Int J Dev Neurosci. 2000;18(1):39–45.
- Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. AnesthAnalg. 2008;106(6):1681–1707.
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits.J Neurosci. 2003;23(3):876–882
- Bhavani-Shankar K, Steinbrook RA, Brooks DC, Datta S. Arterial to end-tidal carbon dioxide pressure difference during laparoscopic surgery in pregnancy. Anesthesiology. 2000;93(2):370–373.
- O’Rourke N, Kodali BS. Laparoscopic surgery during pregnancy.CurrOpinAnaesthesiol. 2006;19(3):254–259.
- Steinbrook RA, Bhavani-Shankar K. Hemodynamics during laparoscopic surgery in pregnancy. Anesth Analg.2001;93(6):1570–1571, table of contents.
- Naughton NN, Cohen SE. Nonobstetric surgery during pregnancy. In: Chestnut DH, editor. Obstetric anesthesia: principles and practice, 3rd ed. Philadelphia: Elsevier Mo s by;2004.p.255-72.
- Azzam FJ, Padda GS, De B o a rd JW, Krock JL, Ko l t e r m a n SM. Preoperative pregnancy testing in adolescents. AnesthAnalg 1996;82:4-7.
- NganKee WD, Khaw KS. Vasopressors in obstetrics: what should we be using? CurrOpinAnaesthesiol 2006;19:238-43.
- August 2022 Obstetric Anaesthetic Guidelines – Cardiff and Vale UHB.
- Odor P.M., Bampoe S., Moonesinghe S.R., et al. General anaesthetic and airway management practice for obstetric surgery in England: a prospective, multicentre observational study. Journal of Maternal-Fetal and Neonatal Medicine. (no pagination). 2021
- https://anaesthetists.org/Portals/0/PDFs/Guidelines%20PDFs/Recommendations%20for%20standards%20of%20monitoring%20during%20anaesthesia%20and%20recovery%202021.pdf?ver=2021-05-26-141701-007.
- Beckett VA, Knight M, Sharpe P. The CAPS Study: incidence, management and outcomes of cardiac arrest in pregnancy in the UK: a prospective, descriptive study. BJOG 2017; 124: 1374–81.
- Mushambi MC, Kinsella SM, Popat M. Obstetric Anaesthetists’ Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia 2015; 70: 1286–306.
- Kasson B, Hledin V, Clayton B et al. Considerations for management of bupivacaine formulation shortage affecting obstetric anesthesia services. AANA J 2018; 86: 76–8.
- Obstetric Anaesthetists Association. OAA commentary on alternatives to intrathecal and epidural diamorphine for caesarean section analgesia.
- Uwubamwen N.A., Verma D., Jones B. Antenatal anaesthetic assessment of obstetric patients. Anaesthesia and Intensive Care Medicine 2022; 23 315-318.