UDK: 618.19-073.432.19
Chabukovska Radulovska J1,2, Petrovska T1,2, Zdarvkovska M2, Danailova M2
1 University Clinic for Surgical Disease “St. Naum Ohridski“, Skopje
2 Faculty of Medical Sciences, “Goce Delchev” University, Shtip
Abstract
Objective: To evaluate the role of breast ultrasound elastography as an emerging sonographic imaging technique that provides additional information in the characterization of solid breast tumors.
Introduction: Breast ultrasound elastography is a non-invasive imaging method that enables the differentiation of solid tumors according to the hardness (elasticity) of the tissue they show. Sonoelastography is used to characterize the lesions previously diagnosed using B-mode ultrasound.
Methods: The study was conducted in 56 patients with a previously diagnosed solid breast lesion with mammography, routine US, and sonoelastography using an ultrasound device (ESAOTE) with a linear multifrequency probe 6-18MHz.
Results: In this study, fifty-six patients were examined by breast ultrasound, sonoelastography and mammography. The findings were confirmed by biopsy. In 45 patients breast lesions were characterized as benign and 11 lesions as suspiciously malignant. The results of our study showed that combined B-mode ultrasound and elastography had 100% sensitivity (CI= 95% 59.04% – 100%) and specificity 91,8 %, (CI = 95% 80.40% – 97.73%) with positive PV 63.64 % (CI = 95% 40.62% – 81.74%) I negative PV 100% (CI = 92.13% – 100%). The accuracy of the reporting method was 92,86% (CI = 95% 82.71% – 98.02%).
Conclusion: Breast sonoelastography is an additional ultrasonographic tool that increases the specificity of conventional B mode US and helps to reduce false-positive results and decrease unnecessary breast biopsies.
Key Words: Breast sonoelastography; solid breast tumors.
Introduction
Elastography (virtual palpation) is a non-invasive ultrasound method that characterizes and differentiates solid tumors, according to the hardness (elasticity) of the tissue they exhibit.
Elastography uses the differences in the mechanical properties of the tissue, in response to the applied force, by determining the indices needed to diagnose and characterize the current state of the change, based on the hardness that the breast tissue shown.
The assessment of tissue hardness has been used for more than a thousand years in diagnostics due to the knowledge that many diseases and conditions lead to changes in the hardness of altered tissues, especially in tumors with an emphasis on malignancy.
Krouskop in 1998 first described the application of sonoelastography in tissue imaging. Sonoelastography of the breast is a method of ultrasound examination of solid changes in the breast, which is used to characterize a lesion already detected in B-mode ultrasound (1).
Sonoelastosonography is generally used for the characterization and differentiation of solid tumors in the breast (Rizzatto et al. 1993) (2).
Elastography is a newer recording technique that assesses the elasticity of tissues after previously obtained information obtained from ultrasound in B-mode (3).
Sonoelastography together with B mode ultrasound, Doppler sonography (color or power doppler) is a part of the multimodal display of tissues and changes in them.
Recent studies show that sonoelastography increases diagnostic accuracy more than conventional B-mode ultrasonography and helps to decrease false-positive results
(increased specificity) and unnecessary breast biopsies (4).
As a result of the type of applied force at the ultrasound elastography technique may be introduced as strain or shear wave elastography.
Strain elastography is a static type of ultrasound elastography which involves tension imaging.
Shear wave elastography is a dynamic type of ultrasound elastography, divided as shear waves and force acoustic radiation pulse recording.
Elastography determines tissue stiffness.
Elastography techniques can be based on strain or wave velocity (strain elastography) or stiffness (Young’s modulus) or modulus from share wave elastography (5). Therefore, elastography techniques can be qualitative by determination of elasticity by color which is coded according to tissue strength, semi-quantitative by determining the strain ratio (SR – the ratio of stiffness in the nodule and the surrounding healthy breast tissue) and quantitative by measurement (SE and SWE) (6).
Strain elastography (static or compression elastography) is qualitative type or semiquantitative by determining the strain ratio (SR) of elastography.
Shear wave (transient elastography) is a quantitative type of elastography.
Strain elastography – historically is the earliest elastography technique, where external tissue compression is applied and comparison between ultrasound images and images after compression. The least deformed are the most rigid areas of the image, and the most deformed areas are the softest (7) (Figure1).
Shear-wave elastography uses waves to assess tissue movement in all directions, and compression is induced deep within the tissue by the acoustic radiation force (8).
Elastography appears to have the best application in solid BI-RADS 3 or 4a lesions, increasing confidence in the diagnosis before biopsy.
Cysts, as anechoic structures are not subject to elastography research, because non-viscous fluids are not compressible, and thus simple cysts do not show deformability signals in elastography techniques (9).
Elastography is based on the fact that in malignant tumors the density of cells increases, which changes the elasticity of the tissue itself. Elastography provides an assessment of the deformation of all tissues (fatty, fibroadenomas, or solid lesions) and shows in real-time that benign nodes such as fibroadenomas, papillomas, etc. (Figure1). In malignant lesions, there is a certain inelasticity of the tissue, which is usually the result of neoplastic infiltration of the interstitium, the desmoplastic reaction intra- and extra-nodular. Such is the case with ductal carcinomas, squamous type of carcinoma. Exceptions are some tumors of the mucinous or papillary carcinoma type which are of low malignant consistency.
Figure1. Elastography shows in real-time benign nodes such as fibroadenomas and papillomas.
In addition to this is the fact that elastography based on the characteristics of the tissue in terms of its stiffness can increase the sensitivity of ultrasound in the preoperative diagnostic period and can be used as an additional diagnostic tool in the prediction of prognostic factors for cancer, taking into account that the higher stiffness values obtained by elastography correlate with weaker parameters of prognostic factors, worse prognosis (10, 11).
The use of elastography in the clinical practice allows for better characterization and categorization of lesions detected by ultrasound in B mode, better categorization in BI RADS classification groups and increasing the diagnostic confidence of positive or negative findings obtained with ultrasound.
Material and Method
At our clinic in 2022, sonoelastography was applied to 56 patients with breast solid tumors. In 18 patients there was information about a positive family history.
Inclusion criteria were patients with solid breast lesions.
Exclusion criteria – Patients with cystic lesions and non-mass lesions were not considered.
All patients underwent an ultrasonographic examination in B-Mode, Color Doppler sonography and Strain sonoelastography. All patients previously had digital mammography.
We use strain elastography as semi-quantitative method with ESAOT/ elastography ultrasound, multifrequency probe 5-18 Mhz.
Strain Elastography (SE) determines tissue stiffness by determining the SR – ratio of stiffness in the nodule to the surrounding healthy breast tissue and is calculated automatically by a built-in software program in the ultrasound device.
In this study evaluating SR, the cut-off value for the differentiation of benign and malignant lesions was 3.
Sonoelastography achieves maximum specificity and accuracy, in ranges between 2 and 4.
For the characterization and evaluation of breast lesions, the elastography characteristics of changes such as size and elasticity were used. A change in the size of solid nodules as a result of the desmoplastic reaction of the tissue which appears larger on elastography than on B-mode ultrasound is in favor of malignant tumors. If this ratio is greater than 1, the lesion is more suspicious to be malignant (12).
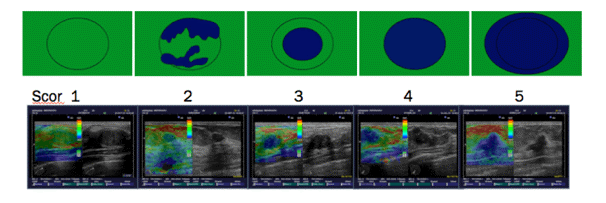
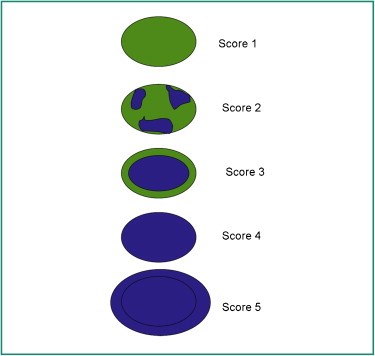
To determine the elasticity of changes, the Tskuba score was used with a rate from 1 to 5 (13, 14) (Figure 2).
Figure 2. The risk of malignancy increases from 1 to 5.
Tskuba score
Score 1: if the lesion is homogeneously elastic, completely green,
Score 2: if the larger lesion is deformable green, and only small parts of the areas are not deformed and are blue,
Score 3: deformable on the periphery of the lesion, and the center is rigid, blue,
Score 4: non-deformable blue lesion,
Score 5: lesion and adjacent tissues are stiff and blue.
According to Tsukuba score, almost all categories 1-3 are benign, and categories 4 and5 are malignant.
Invasive cancer has the lowest elasticity, followed by non-invasive cancer, fibrous tissue, normal structural tissue, and finally breast fat shows the highest elasticity.
The classification was also done using BI RADS classification groups.
A categorization of the lesions in BI RADS according to their shape, edges, orientation, vascularity and echogenicity was done. According to ultrasonographic elastography criteria, lesions were classified as soft, medium and hard changes.
All patients underwent a core biopsy of the solid nodules.
Results
With sonoelastosonography, 56 patients were examined.
In 44 patients the changes in the breast were characterized as benign, and in 12 patients with elastosonography, the changes were co-characterized as a suspected malignant lesion.
According to BI RADS classification, 44 patients were in BI RADS 3 Classification Group, in BI RADS 4 Classification Group there were 9 patients, and 3 patients were in BI RADS 5 Classification Group.
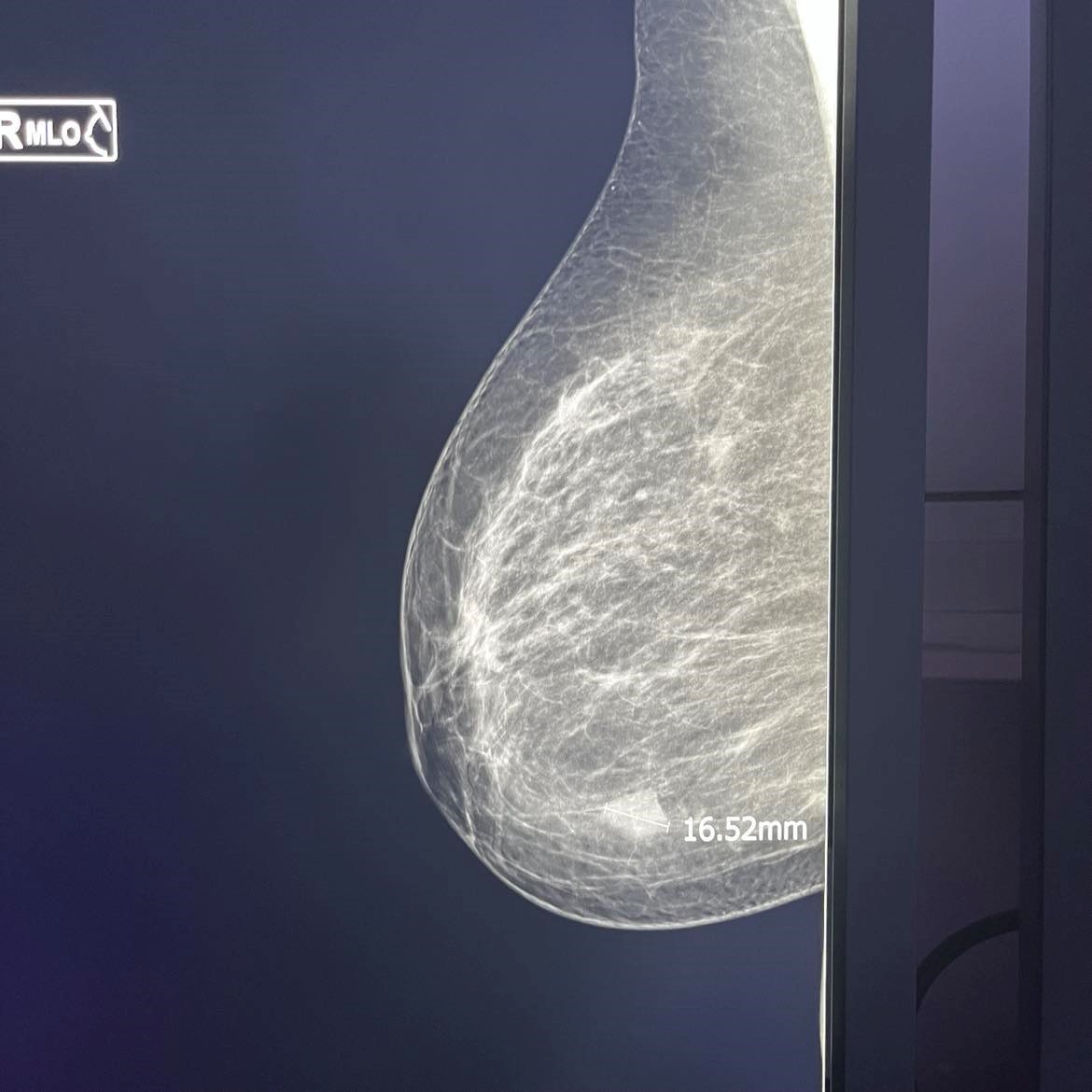
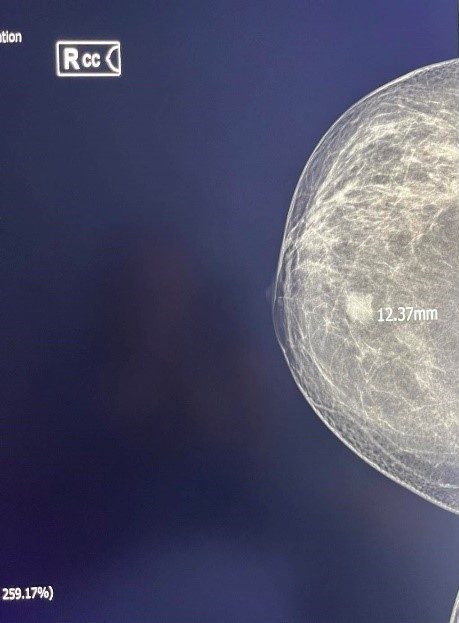
Figure 3. Inv. Intraductal Breast Carcinoma-MG.
Figure 4. Inv. Intraductal Breast Carcinoma (same patient) – Sonoelastography, US
According to Cito/ptx, 49 changes were found to be benign, and 7 of the changes were more likely to be malignant, Table1.
After the obtained pathohistological results, in 5 patients the classification group was changed from BI RADS 4 to BI RADS 3. In 4 patients in whom the BI RADS classification group was changed from BI RADS 4 to BI RADS 3, the pathohistological findings were in the direction of atypical fibroadenomas with calcifications, in one patient the finding is a radial scar lesion.
Table 1. Results from Elastography (El.Index), Cito/Pathology.
| Elastic Index | Ct/Pth | Total number | |
| ( + ) | ( – ) | ||
| ( + ) | 7 | 4 | 11 |
| ( – ) | 0 | 45 | 45 |
| Total number | 7 | 49 | 56 |
Se = 100% – Sensitivity (CI = 95% 59.04% – 100%)
Sp = 91.8% Specificity (CI = 95% 80.40% – 97.73%)
+ PV = 63.64% Positive pred. Value (CI = 95% 40.62% – 81.74%)
– PV = 100% Negative pred. Value (CI = 92.13% – 100%)
Accuracy = 92,86% (CI = 95% 82.71% – 98.02%).
Discussion
According to the literature, invasive cancer has the lowest elasticity, followed by non-invasive cancer, fibrous tissue, normal structural tissue, and finally breast fat shows the highest elasticity (15).
The multicenters study which Barr. et al. performed on 635 lesions (413 benign, 222 malignant, confirmed by cytology) resulted in a sensitivity of elastography of 98.6% and a specificity of 87.4%. There was also a variation of sensitivity between 96.7% and 100% and specificity between 66.7% and 95.4% in the different centers participating in the study.
Regarding the high sensitivity in lesion characterization, there is inter-operator variability between different centers that is related to individual differences in examination technique, suggesting that better standardization of protocols and performances is needed (16).
In the study by Tozaki et al. performed on 100 patients, using Young’s modulus in the differentiation of benign from malignant lesions in elastography, yielded sensitivity values of 91.3% and specificity of 80.6% (17, 18).
In the study by Zhi et al. conducted on 296 patients with dense breasts, after comparing the changes in RTE, US and mammography, and the differentiation of breast changes in benign and malignant groups, according to the Itoh scoring system, elastography has the highest specificity of 95.7% and the lowest rate of false positives of 4.3% compared to ultrasound and mammography (19).
According to our experience elastography shows high sensitivity 100% with CI= 95% (59.04% -100%) and specificity 91,8% with CI=95 (89,40% – 97,73%) regarding the characterization of lesions. Accuracy = 92, 86%, CI=95% (82,72%- 98,02).
Despite the high sensitivity and specificity, elastography shows certain advantages and disadvantages.
The disadvantages mainly refer to the fact that all malignant tumors are not rigid, taking into account the different histopathological forms of cancer to avoid false negative or false positive results.
The greatest advantage of elastography is the high sensitivity regarding characterization and assessment of solid breast lesions which increases the sensitivity of ultrasound in detecting the malignant tumors and positive lymph nodes in breast cancer, which is especially important in the preoperative diagnostic period (20,21).
The medical device industry is launching a series of advanced ultrasound devices and software intended for elastography. Realizing the benefits of elastography as an added value to ultrasound, EFSUMB (European Federation of Societies for Ultrasound in Medicine and Biology) and WFUMB (World Federation for Ultrasound in Medicine and Biology) have issued a guide to these innovations in ultrasound diagnostics and clinical application of elastography (22.23).
Conclusion
Sonoelastography is a new diagnostic modality for examining and characterizing solid tumors in the breast that cannot completely replace other imaging methods but is an excellent complementary method in the detection and differentiation of solid breast lesions. Sonoelastography is a tool to help characterize changes in the breast, it is not a detection tool. Sonoelastography is an example of the added value of ultrasound and in accordance with the so-called multimodal US approach improves the specificity of ultrasound, increases the confidence of the diagnostician, and can reduce the number of unnecessary biopsies and patient’s anxiety.
References
- Ophir J, Alam SK, Garra B, et al. Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. Proc Inst Mech Eng H. 1999; 213(3):203-33. doi: 10.1243/0954411991534933. PMID: 10420776.
- Rizzatto G, Aiani L, Baldassarre S, et al. Characterization of breast lesions with real-time sonoelastography: results from the Italian multicenter clinical trial. 2006. Abstract-RSNA. Chicago, USA.
- Imtiaz S. (Mar 08, 2018). Breast elastography: A new paradigm in diagnostic breast imaging. Appl Radiol. 2018; 47(3):14-19.
- Elkharbotly A, Farouk HM. Ultrasound elastography improves differentiation between benign and malignant breast lumps using B-mode ultrasound and color Doppler. Egyptian Journal of Radiology and Nuclear Medicine. 2015. 46;(4):1231–1239.
- Xiao Y, Zeng J, Zhang X et al. Ultrasound strain Elastography for breast lesions: computer-aided evaluation with quantifiable Elastographic features. J Ultrasound Med. 2017;36(6):1089–100.
- Zhi H, Xiao XY, Yang HY, et al. Semi-quantitating stiffness of breast solid lesions in ultrasonic elastography. AcadRadiol. 2008; 15:1347–1353. doi:10.3969/j.issn.2095-3941.2012.02.008.
- Wang Z, Yang T, Wu Z, et al. Correlation between elastography score and strain rate ratio in breast small tumor.Zhong Nan Da XueXueBao Yi Xue Ban. Journal of Central South University Medical Sciences. 2010; 35:(9):928–932.
- Yang H, Xu Y, Zhao Y, Yin J, Chen Z, Huang P. The role of tissue elasticity in the differential diagnosis of benign and malignant breast lesions using shear wave elastography. BMC Cancer. 2020 Sep 29; 20(1):930. doi: 10.1186/s12885-020-07423-x. PMID: 32993571; PMCID: PMC7526131.
- Destounis S, Arieno A, Morgan R, et al. Clinical experience with elasticity imaging in a community-based breast center. J Ultrasound Med. 2013; 32(2):297–302.
- Stavros AT, Thickman D, Rapp CL, et al. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995; 196(1):123–134.
- Goddi A, Bonardi M, Alessi S. Breast elastography: a literature review. J Ultrasound. 2012; 15:(3):192–198.
- Kim MY, Cho N, Yi A, et al. Sonoelastography in distinguishing benign from malignant complex breast mass and making the decision to biopsy. Korean J Radiol. 2013; 14(4):559–567. doi:10.3348/kjr.2013.14.4.559.
- Balleyguier C, Ciolovan L, Ammari S, et al. Breast elastography: the technical process and its applications. Diagn Interv Imaging. 2013 May; 94(5):503-13. doi: 10.1016/j.diii.2013.02.006. Epub 2013 Apr 22. PMID: 23619293.
- Dawood, M. A. E., Ibrahim, N. M. A., Elsaeed, H. H., & Hegazy, N. G. (2018). Diagnostic performance of sonoelastographic Tsukuba score and strain ratio in evaluation of breast masses. The Egyptian Journal of Radiology and Nuclear Medicine, 49(1), 265-271. https://doi.org/10.1016/j.ejrnm.2017.10.005.
- Barr RG. Sonographic breast elastography: a primer. J Ultrasound Med. 2012; 31(5):773–783.
- Barr RG, Destounis S, Lackey LB, Svensson WE, Balleyquier C, Smith C. Evaluation of breast lesions using sonographic elasticity imaging: a multicenter trial. J Ultrasound Med. 2012; 31(2):281–7.
- Tozaki M, Fukuma E. Pattern classification of shear wave Elastography images for differential diagnosis between benign and malignant solid breast masses. Acta Radiol. 2011; 52:1069–75.
- Tozaki M, Isobe S, Sakamoto M. Combination of elastography and tissue quantification using the acoustic radiation force impulse (ARFI) technology for differential diagnosis of breast masses. Jpn J Radiol. 2012; 30(8):659–670.
- Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006; 239:341–50.
- 3. Kim EK, Ko KH, Oh KK, et al. Clinical application of the BI-RADS final assessment to breast sonography in conjunction with mammography. AJR Am J Roentgenol. 2008; 190(5):1209–15.
- Sadigh G, Carlos RC, Neal CH, Dwamena BA. Ultrasonographic differentiation of malignant from benign breast lesions: a meta-analytic comparison of elasticity and BIRADS scoring. Breast Cancer Res Treat. 2012; 133(1):23–35.
- Shiina T, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 1: basic principles and terminology. Ultrasound Med Biol. 2015; 41(5):1126–47.
- Fleury EF. The importance of breast elastography added to the BI-RADS (5th edition) lexicon classification. Rev Assoc Med Bras. 2015;61(4):313-316.