Kiprijanovska B1, Georgieva D1,2, Kuzmanovska B1,2, Nancheva J1,2, Tolevska M1,2, Panov S3
1University Clinic for TOARILUC, Skopje, Republic of North Macedonia
2Medical Faculty,” Ss. Cyril and Methodius” University, Skopje, Republic of North Macedonia
3Institute of Biology, Faculty of Natural Sciences and Mathematics, “Ss. Cyril and Methodius” University, Skopje, Republic of North Macedonia
DOI: https://www.doi.org/10.55302/MJA2373034k
Abstract
Background: The relationship between genetic predisposition and the development of postoperative delirium has not yet been established. The e4 allele of the apolipoprotein E gene has been reported as a genetic risk factor for delirium.
Objective: This paper analyzed the relationship between the frequency of genotypes of the APOE rs7412/rs429358 polymorphism, which contains the minor allele e4, and the occurrence of postoperative delirium.
Material and Methods: The study included patients aged 65 years and older without pre-existing cognitive impairment admitted to the University Clinic for Traumatology and Orthopedics for operative treatment of a fracture of the upper end of the thighbone. The Confusion Assessment Method (CAM) confirmed the delirium diagnosis. APOE rs7412/rs429358 polymorphism genotypes were determined by molecular genetic analysis using the quantitative real-time amplification method (qRT-PCR) on DNA samples extracted from venous blood leukocytes.
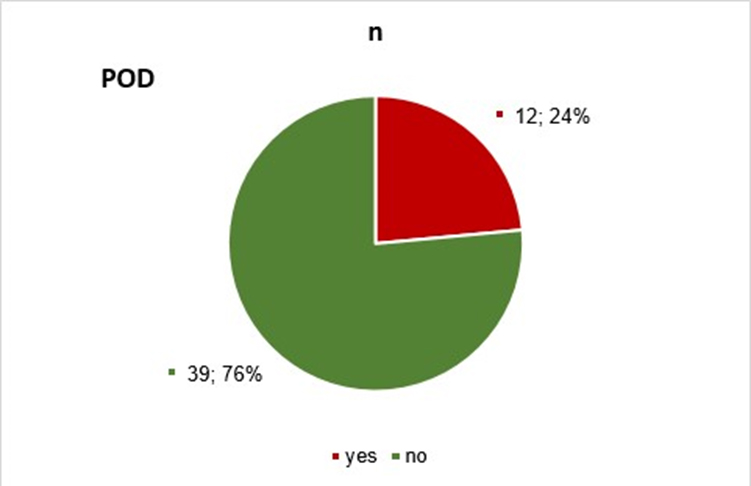
Results: The presented results are from analyzed samples and data from 51 patients. Out of these, postoperative delirium was diagnosed in 12 patients, while in 39 patients weren’t registered, and they are the control group in the trial.
Conclusion: This study results indicate the association of the studied polymorphism in the apolipoprotein E gene, which contains the minor allele e4, with the occurrence of postoperative delirium in this group of adult patients. A larger group is necessary to reach more valid conclusions.
Key Words: adult patients, apolipoprotein E, genotype, postoperative delirium.
Introduction
Postoperative delirium (POD) occurs frequently in elderly patients, with an incidence that varies widely from 20% to 55% after high-risk procedures that include vascular, orthopedic and cardiac operations (1-4). A meta-analysis of 26 studies of postoperative delirium presented an incidence of 4.0 to 53.3% in patients with hip fractures and 3.6 to 28.3% in elective orthopedic surgeries (5). Postoperative delirium has adverse outcomes, such as increased morbidity and mortality, cognitive and functional decline leading to loss of autonomy and reduced quality of life, prolonged hospital stay, institutionalization and additional health care costs. Patients with postoperative delirium may be at risk of developing long-term postoperative cognitive dysfunctions and the onset of dementia (6,7).
The pathogenesis of POD is not yet clearly understood. However, there are many evidences from animal and human studies regarding the underlying processes behind this clinical syndrome. Maldonado’s landmark review described that potential mechanisms can be grouped into two categories: neuroinflammation and oxidative stress, which likely interact by promoting neurotransmitter dysregulation, and neuronal network dysfunction (8). The neuroinflammatory theory suggests that several noxious stimuli, such as surgical stress and infection, trigger the activation of the inflammatory cascade with the acute release of inflammatory mediators into the bloodstream. Acute peripheral inflammatory stimulation induces activation of brain parenchymal cells (microglia and astrocytes) and expression of proinflammatory cytokines and inflammatory mediators in the CNS. These neuroinflammatory changes cause neuronal and synaptic dysfunction and subsequent neurobehavioral and cognitive symptoms. The oxidative stress hypothesis proposes that brain hypoperfusion induces local ischemia that triggers a chain of events. First, there is increased production of reactive oxygen products, and then the reactive oxygen products lead to excitotoxicity, apoptosis and local inflammation.
Apolipoproteins cannot cross the blood-brain barrier but are still expressed in the central nervous system (CNS) by astrocytes, microglia and oligodendrocytes, where they transport phospholipids and cholesterol for neuronal membrane regeneration and remyelination. There are three ApoE isoforms – E2, E3 and E4 derived from the apolipoprotein E gene allelic variants. Apolipoprotein E (ApoE) is involved in recovering the central nervous system from injury. Apolipoprotein E regulates normal neuronal function through cholesterol transport and cell repair (9). The APOE gene is located on the long arm of chromosome 19. It has the chromosomal location 19q13.2 and encodes apolipoprotein E. The protein itself can be in one of three major isoforms, encoded by variant alleles that depend on the nucleotide sequence of the polymorphic positions rs429358 and rs7412 in the fourth exon of the APOE gene. According to the amino acid residues encoded by codons 112 and 158, the three isomorphs of apolipoprotein E are distinguished, as shown in Table 1.
Table 1. Nucleotide and amino acid sequences in the studied polymorphisms in the APOE gene
| APOE-alleles | polymorphisms | codons | ||
| Rs429358 | Rs7412 | 112 | 158 | |
| ε2 | T | T | Cys | Cys |
| ε3 | T | C | Cys | Arg |
| ε4 | C | C | Arg | Arg |
Depending on the presence of the ε2, ε3, and ε4 alleles at the APOE gene locus, each individual may have one of the following six genotypes: ε2/ε2, ε3/ε3, ε4/ε4, ε2/ε3, ε2/ε4 and ε3.
ApoE4 is more vulnerable to degradation than other isoforms, thereby limiting lipid mobilization for repair. Hypothesized pathophysiological mechanisms that explain the relationship between ApoE and delirium, include modulation of brain inflammatory response and modification of glial activation, blocking nicotinic acetylcholine receptors, causing an anticholinergic effect that has been hypothesized in delirium, and suppression of cerebral glucose metabolism in APOE ε4 carriers may represent another pathophysiological mechanism. In already damaged brains, the presence of the APOE ε4 allele accelerates the promotion of Aβ aggregation and fibril formation, leading to the progression of dementia (10-12).
The ε4 allele of the apolipoprotein E gene has been hypothesized as a genetic risk factor for delirium.
Material and Methods
This study included 51 patients who underwent emergency surgery due to a fracture of the upper end of the thighbone (femur), aged 65 and older, and ASA groups I, II, or III. Patients with cognitive impairment, dementia or other neurodegenerative diseases, stroke with the residual deficit, use of drugs that affect cognitive functions, abuse of alcohol and drugs, blindness, deafness, contraindication for spinal anesthesia, and admission to the intensive care unit were not taken into the study. We assessed preexisting cognitive impairment with two validated instruments, the Short Mental Test (AMT 10) (13,14) and the Cognitive Decline Information Questionnaire – Short Form (IQCODE ‐ SF) (15). Screening for POD began immediately after surgery and was monitored throughout the hospital stay with the Confusion Assessment Method (CAM) (16).
From patients who met the inclusion criteria for the study, we collected a 3mL venous blood sample with the anticoagulant EDTA-Na2 (disodium salt of ethylenediamine tetraacetate) in a vacuum test tube. Samples were taken only from patients who, after a detailed explanation of the procedure, the goals and their rights, signed a written consent.
The APOE rs7412/rs429358 polymorphism genotypes were determined by molecular genetic analysis using the quantitative real-time amplification method (qRT-PCR) on DNA samples extracted from venous blood leukocytes from patients. Amplification curves were analyzed with the StepOne software (Applied Biosystems) using the allelic discrimination method, which determined the APOE rs7412/rs429358 polymorphism genotype.
The association of the existence of postoperative delirium with the genotypic and allelic frequency of the APOE rs7412/rs429358 polymorphism in patients was analyzed by Pearson’s Chi-square test and Fisher’s exact test. According to these data, the odds ratio was also calculated. The CI (confidence interval) calculations are performed at 95%, that is, at p<0.05. Values of p<0.05 are considered statistically significant, while those with p<0.01 are considered highly significant. Statistical calculations were performed using XLSTAT 2016 and GenAlEx 6.5 software add-ins installed on Microsoft Excel 2016.
Results
This paper presents the results of the analysis of samples and data from 51 patients who underwent surgery on the upper end of the femur. Postoperative delirium was diagnosed in 12 patients, while it wasn’t registered in 39 patients, and they are the control group in the trial (Table 1 and Graph 1).
Table 1. Prevalence of postoperative delirium in patients,
| POD | n | % |
| Yes | 12 | 23.53 |
| No | 39 | 76.47 |
| Total | 51 | 100.00 |
Chart 1. Prevalence of postoperative delirium in patients.
The data on gender and age distribution in the studied two groups of patients (the group with registered postoperative delirium and the control group) are shown in Tables 2 and 3 and in Charts 2 and 3.
Table 2. Gender distribution of the two groups of patients.
| Gender | Group with POD | Control group | Fisher’s exact test | ||
| n | % | n | % | p | |
| male | 3 | 25.00 | 9 | 23.08 | 1.000 |
| female | 9 | 75.00 | 30 | 76.92 | |
| total | 9 | 100.00 | 39 | 100.00 | |
Chart 2. Gender distribution of the two groups of patients.
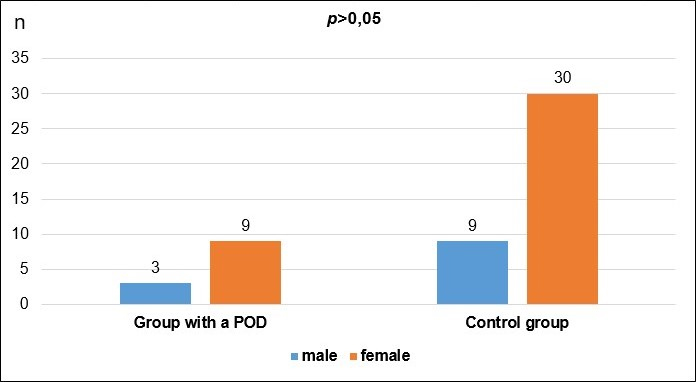
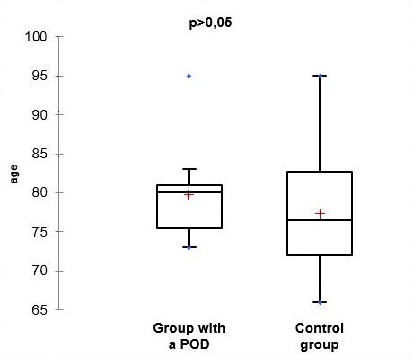
Table 3. Age structure of the two groups of patients.
| Parameter (years) | Group with POD | Control group | Student t-test |
| n | 12 | 39 | 0.323 |
| average | 79.82 | 77.37 | |
| SD | 6.23 | 7.18 | |
| min. age | 73 | 66 | |
| max. age | 95 | 95 | |
From the presented data and the results of the statistical analysis of the gender and age structure of the patients, we can conclude that the differences between the two groups do not have statistical significance (p>0.05). This comparison means that the two groups are balanced by gender and age, facilitating their further comparison.
Chart 3. Age structure of the two groups of patients.
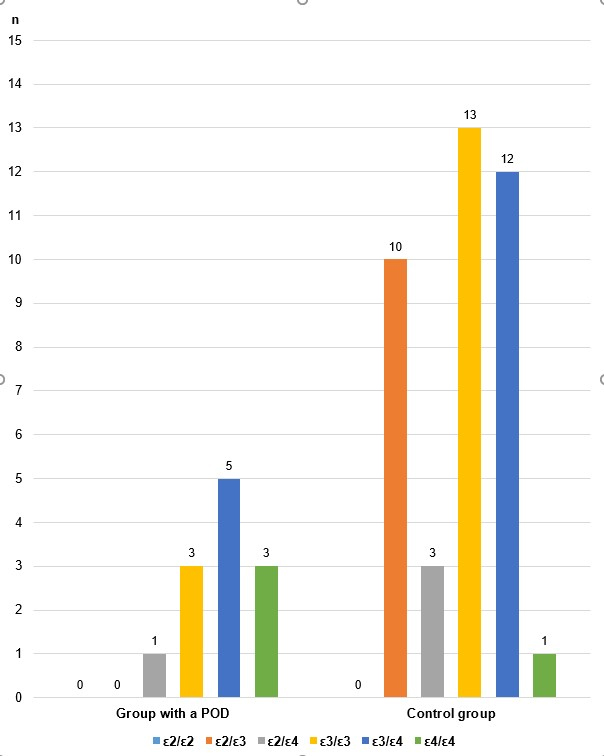
The results of the analyses obtained by determining the genotypes of the rs7412/rs429358 polymorphism in the APOE gene are shown in Table 4 and Charts 4 and 5.
Table 4. APOE rs7412/rs429358 genotypes in both groups of patients
| Group | APOE rs7412/rs429358 | n | % |
| Group with POD | ε2/ε2 | 0 | 0.00 |
| ε2/ε3 | 0 | 0.00 | |
| ε2/ε4 | 1 | 8.33 | |
| ε3/ε3 | 3 | 25.00 | |
| ε3/ε4 | 5 | 41.67 | |
| ε4/ε4 | 3 | 25.00 | |
| Total | 12 | 100.00 | |
| Control group | ε2/ε2 | 0 | 0.00 |
| ε2/ε3 | 10 | 25.64 | |
| ε2/ε4 | 3 | 7.69 | |
| ε3/ε3 | 13 | 33.33 | |
| ε3/ε4 | 12 | 30.77 | |
| ε4/ε4 | 1 | 2.56 | |
| Total | 39 | 100.00 |
Chart 4. APOE rs7412/rs429358 polymorphism genotypes in both groups of patients.
Graph 5. Alleles of the APOE rs7412/rs429358 polymorphism in both groups of patients.
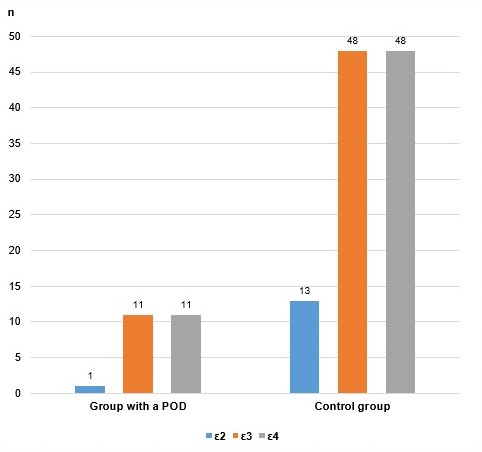
Due to the relatively small number of cases analyzed in this paper, we evaluated the existence of a genetic association of the investigated polymorphism with postoperative delirium by combining the genotypes and alleles according to whether they contain the ε4 allele, with the modified genotypic, as well as with the allelic and additive model. The results are shown in Table 5.
Table 5. Genetic-association analysis.
| Genetic model | APOE rs7412/rs429358 genotype/allele comb. | Group with POD | Control group | | pp | OR (95% CI) | ||
| nn | %% | nn | x% | |||||
| Genotypic | ε2/ε2 + ε2/ε3 + ε3/ε3 | 33 | 225.00 | 223 | 58.97 | rref. | rref. | ref. |
| ε2/ε4 + ε3/ε4 + ε4/ε4 | 99 | 775.00 | 116 | 41.03 | 44.238 | 00.040 | 4.313 (1.007 – 18.461) | |
| Total | 112 | 1100.00 | 339 | 100.00 | ||||
| Allelic | ε2 + ε3 | 112 | 550.00 | 661 | 78.21 | 77.175 | 00.007 | 3.588 (1.368 – 9.409) |
| ε4 | 112 | 550.00 | 117 | 21.79 | ||||
| Total | 224 | 1100.00 | 778 | 100.00 | ||||
| Additive | 0 ε4 | 33 | 225.00 | 223 | 58.97 | 22.374 | 00.018 | / |
| 1 ε4 | 66 | 550,00 | 115 | 38.46 | ||||
| 2 ε4 | 33 | 225.00 | 11 | 2.56 | ||||
| Total | 112 | 1100.00 | 339 | 100.00 | ||||
According to the obtained calculations, the frequency of combinations of genotypes that do not contain the ε4 allele (ε2/ε2 + ε2/ε3 + ε3/ε3) is significantly higher (58.97%) in the control group, as opposed to a lower representation (25%) in patients with postoperative delirium. This genotype was used as a reference for the calculation according to the modified genotypic model.
On the contrary, the frequency of combinations of genotypes containing the ε4 allele (ε2/ε4 + ε3/ε4 + ε4/ε4) is significantly higher in patients with postoperative delirium (75%) compared to control patients in whom no delirium was registered (41.03 %). This difference is statistically significant (p<0.05) with the Chi-square test and confirmed with Fisher’s exact test.
The calculated probability index OR is 4.313. Statistically, such value implies that the carriers of one of the genotypes containing the ε4 allele (ε2/ε4 + ε3/ε4 + ε4/ε4) have a 4.3 times higher probability of developing postoperative delirium during traumatological operations, compared to patients who are carriers of a genotype that does not contain the ε4 allele (ε2/ε2 + ε2/ε3 + ε3/ε3). This difference is statistically significant (p=0.040). The 95% confidence interval ranges from 1.007 to 18.461.
Analysis with the allelic model also supports a statistically significant association of the presence of the ε4 allele with an increased probability of postoperative delirium. According to this genetic-associative model, carriers of the ε4 allele have a 3.58 times higher chance of developing this complication than patients who do not carry the ε4 allele. In this calculation, the difference is statistically significant (p=0.007). The confidence interval at 95% is in the range of 1.368 to 9.409.
Further genetic analysis was performed with the additive model, comparing the frequencies of individuals who are not carriers of the ε4 allele (marked as 0 ε4), than of those who are carriers of one ε4 allele (1 ε4), i.e., of two ε4 alleles (2 ε4).
The calculations performed with the Cochran-Armitage ordinal test show a statistically significant genetic association of the ε4 allele with postoperative delirium (p<0.05). Odds ratio (OR) calculation is not possible with this model.
Discussion
In this paper, our interest was focused on the analysis of the association of the occurrence of postoperative delirium with the polymorphism in the apolipoprotein E gene, which contains the minor allele e4, among elderly patients admitted to the medical department of the Clinic for Traumatology and the Clinic for Orthopedics, for operative treatment of the upper end of the femur with spinal anesthesia. The protocol didn’t mandate the management of spinal anesthesia to maximize the external validity of the results. To examine preexisting cognitive dysfunction in patients, we used two validated instruments, the Short Mental Test (AMT 10) (13,14) and the Cognitive Decline Information Questionnaire – Short Form (IQCODE ‐ SF) (15). The Short Mental Test (AMT – 10) is a relevant tool for assessing mental status in emergency medicine departments, especially useful for rapid assessment of elderly patients for possible dementia. For the diagnosis of postoperative delirium in patients after surgery and anesthesia, we used the Confusion Assessment Method (CAM), which is the most widely used instrument for the identification of delirium by non-psychiatric personnel, developed using criteria adapted from the Diagnostic and Statistical Manual of Mental Disorders fifth edition (DSM-V). The CAM has high specificity (89%) and sensitivity (94%) compared to delirium ratings by geriatric psychiatrists (17,18). We should exclude other neurocognitive disorders to confirm the diagnosis of delirium. Also, we should carefully monitor the effect of sedative drugs in evaluating delirium. Given the high prevalence of delirium in older patients and the adverse clinical outcome, current clinical practice guidelines recommend routinely screening patients for delirium using a validated screening tool. Screening of at-risk patients may improve recognition of delirium, allowing for early intervention that can potentially reduce duration and complications (19).
In this paper, we analyzed samples and data from 51 patients, out of which postoperative delirium was diagnosed in 12 patients. At the same time, it was not registered in 39 patients, and they are the control group in the study. Both groups were balanced for gender and age, facilitating their further comparison. Due to the relatively small number of analyzed cases, we evaluated the existence of a genetic association of the examined polymorphism with postoperative delirium by combining the genotypes and alleles according to whether they contain the ε4 allele with the modified genotypic, as well as with the allelic and additive model.
The results in this paper indicate an association between the frequency of genotypes of the studied polymorphism in the APOE gene containing the minor allele ε4 and the occurrence of delirium after surgery and anesthesia. Carriers of the ε4 allele have a 20-fold higher probability of developing postoperative delirium than those of a genotype that does not contain the ε4 allele.
The relationship between genetic predisposition and the development of postoperative delirium has not yet been established in the literature dealing with this problem. While some have found that the presence of the ε4 allele of the apolipoprotein E (APOE4) gene increases the risk of POD (20,21), others have not reached such a conclusion (22). Meta-analyses (23,24) showed a longer duration, but not a more frequent occurrence of delirium.
The obtained knowledge can potentially be applicable in basic science and prospectively in clinical application by designing better prevention and treatment strategies in this patient population.
Conclusion
The results in this paper indicate a genetic association of the studied polymorphism in the APOE gene with the occurrence of postoperative delirium in adult patients. Carriers of the ε4 allele have a 20-fold higher probability of developing postoperative delirium during emergency operations for fracture of the upper end of the femur compared to patients who are carriers of the genotype that does not contain the ε4 allele. A larger group is necessary to reach more valid conclusions.
References:
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet 2014; 383:911–22.
- Abate SM, Checkole YA, Mantedafro B, et al. Global prevalence and predictors of postoperative delirium among non-cardiac surgical patients: a systematic review and meta-analysis. Int J Surg Open 2021; 32:100334.
- Mosk CA, Mus M, Vroemen JP, et al. Dementia and delirium, the outcomes in elderly hip fracture patients. Clin Interv Aging 2017; 12:421–30.
- van der Mast RC, Roest FH. Delirium after cardiac surgery: a critical review. J Psychosom Res. 1996; 41(1):13–30. (PubMed: 8887815).
- Bruce AJ, Ritchie CW, Blizard R, et al. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatr 2007; 19:197–214.
- Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010; 304(4):443–451. (PubMed: 20664045).
- Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012; 367:30–9.
- Maldonado J. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013; 21:1190–1222. (PubMed: 24206937)
- Benarroch EE: Brain cholesterol metabolism and neurologic disease. Neurology 2008; 71:1368–1373.
- Adamis D, Treloar A, Martin FC, et al: APOE and cytokines as biological markers for recovery of prevalent delirium in elderly medical inpatients. Int J Geriatr Psychiatry 2007; 22:688–694.
- Jagust WJ, Landau SM: Apolipoprotein E, not fibrillar β-amyloid, reduces cerebral glucose metabolism in normal aging. J Neurosci 2012; 32:18227–18233.
- Caplan GA, Kvelde T, Lai C, et al: Cerebrospinal fluid in long-lasting delirium compared with Alzheimer’s dementia. J Gerontol A Biol Sci Med Sci 2010; 65:1130–1136.
- Hodkinson, HM (1972). “Evaluation of a mental test score for assessment of mental impairment in the elderly.”. Age and Ageing 1 (4): 233-8. PMID 4669880. http://ageing.oxfordjournals.org/cgi/reprint/1/4/233.
- Qureshi KN, Hodkinson HM. (1974) Evaluation of a ten-question mental test in the institutionalized elderly. Age & Ageing. 3, 152–157.
- Jorm A: The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): a review. Int Psychogeriatr 2004;16:275–293.
- Inouye SK, Kosar CM, Tommet D, et al. The CAM-S, a new scoring system for delirium severity: Association with clinical outcomes. Ann Intern Med. 2014; 150:526–533. (PubMed: 24733193).
- Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The confusion assessment m. ethods. A new method for detection of delirium. Ann Intern Med. 1990; 113:941–948. (PubMed: 2240918).
- Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008; 56:823–830. (PubMed: 18384586)
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet 2014; 383:911–22.
- Leung JM, Sands LP, Wang Y, et al. Apolipoprotein E e4 allele increases the risk of early postoperative delirium in older patients undergoing noncardiac surgery. Anesthesiology. 2007; 107(3):406–411. (PubMed: 17721242).
- van Munster BC, Korevaar JC, Zwinderman AH, et al. The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta-analysis. Am J Geriatr Psychiatry. 2009; 17(10):856–862. (PubMed: 19910874).
- Bryson GL, Wyand A, Wozny D, et al. A prospective cohort study evaluating associations among delirium, postoperative cognitive dysfunction, and apolipoprotein E genotype following open aortic repair. Can J Anaesth. 2011; 58(3):246–255. (PubMed: 21222188.
- van Munster BC, Korevaar JC, Zwinderman AH, et al: The association between delirium and the apolipoprotein E ε4 allele: new study results and a meta-analysis. Am J Geriatr Psychiatry 2009;17:856–862.
- Adamis D, Meagher D, Williams J, et al: A systematic review and meta-analysis of the association between the apolipoprotein E genotype and delirium. Psychiatr Genet 2016; 26:53–59.