Kolevska S1, Gavrilovska Brzanov A2, Kartalov A2, Panovska Petruseva A2, Stavridis S3, Jovanovski-Srceva M2
1City General Hospital “GOB 8th of September”, Skopje, Republic of North Macedonia
2University Clinic for Traumatology, Orthopedic Diseases, Anesthesia,
Reanimation, Intensive Care and Emergency Centre, Medical Faculty, University “Ss. Cyril and Methodius,” Skopje, Republic of North Macedonia
3University Clinic of Urology, Medical Faculty, University “Ss. Cyril and Methodius,” Skopje, Republic of North Macedonia
DOI: https://www.doi.org/10.55302/MJA2373089k
Abstract
Introduction: The clinical presentation of pheochromocytoma varies depending on the location and extent of catecholamine secretion. Whenever possible, surgery is the preferred treatment for these tumors and can cure more than 90% of the patients. However, managing these patients requires a collaborative approach involving multiple medical specialists. Increased awareness of the importance of optimizing patients’ symptoms and conditions before surgery has resulted in established procedures.
Aim: We want to present a case where, based on laboratory investigations, the patient did not have any concessions, although a biopsy of the adrenal gland due to clinical symptoms revealed increased secretion of adrenalin and aldosterone. Also based on the patient’s clinical presentation during surgery, the patient’s symptoms, along with the results of the imaging and intraoperative manifestations, resembled those that are typically associated with pheochromocytoma.
Material and Methods: In this report, we present a management of a case of active adrenal gland adenoma in a young, 18-years-old female with a history of paroxysmal hypertension, palpitations, and excessive flushing of the face who underwent adrenal gland biopsy and was diagnosed with an adrenal gland tumor and underwent left adrenalectomy, resulting in a favorable outcome.
Results: Biochemical tests were within normal ranges, except for a slightly increased CRP of 51.64mg/L, Direct Renin Immunoassay (CLIA) 56ng/l and Noradrenalin 514ng/L. A sampling of the adrenal gland showed hyperfunction on the cortex of the adrenal gland: cortisol 3.3μ/dL, aldosterone 443ng/dL, and adrenalin 333pg/mL.
Conclusion: Early diagnosis, multidisciplinary collaboration, appropriate preoperative medical management and prompt surgical intervention are crucial in reducing morbidity and mortality and preventing complications such as cardiovascular disease in patients with active adrenal glands.
Key Words: adrenal gland adenoma, hypertension, Pheochromocytoma, venous sampling.
Introduction
Pheochromocytoma is a rare tumor that affects only one to four out of every million people each year. It can cause high blood pressure. Even though it causes hypertension in less than 1% of the cases, it is very important to think about this tumor when evaluating people with hypertension. Pheochromocytoma arises from chromaffin cells in the embryonic neural crest that produce catecholamines. These cells are primarily located in the adrenal medulla, but they can also occur in other areas, known as paragangliomas (1).
The clinical presentation of pheochromocytoma varies depending on the location and the extent of catecholamine secretion and may include the classic symptoms of hypertension, headaches, palpitations and excessive sweating. Diagnosing pheochromocytoma requires detecting excessive catecholamine release and documenting the anatomical location of the tumor (1).
When possible, surgery is the preferred treatment for these tumors and can cure more than 90% of the patients. However, managing these patients requires a collaborative approach involving multiple medical specialists, such as endocrinologists, surgeons, cardiologist and anesthesiologists. Increased awareness of the importance of optimizing patients’ symptoms and conditions before surgery has resulted in established procedures. Significant advancements in surgical and anesthetic techniques have also played a significant role in reducing the historical morbidity and mortality rates of patients undergoing surgical tumor removal (2,3).
We want to present a case where, based on laboratory investigations, the patient did not have any concessions, although a biopsy of the adrenal gland due to clinical symptoms revealed increased secretion of adrenalin and aldosterone. as Also, based on the patient’s clinical presentation during surgery, the patient’s symptoms, along with the results of imaging and intraoperative manifestations, it was resembled of those typically associated with pheochromocytoma.
Material and Method
In this report, we present the medical management of a case of an active adrenal gland in a young, 18-years-old female, non-smoker with a BMI of 24.2., with a history of paroxysmal hypertension, palpitations and excessive flushing of the face who underwent adrenal gland biopsy and was diagnosed with an adrenal gland tumor. She underwent a left supra-adrenalectomy, resulting in a favorable outcome.
The patient was admitted to the Clinic of Urology. Because of paroxysmal attacks of hypertension, and in order to avoid damage to target organs, and taking into account the age of the patient who plans to become pregnant and give birth, surgical intervention with a supra-adrenalectomy was indicated with consent of the patient.
Our patient reported episodes of hypertensive crisis a few months before hospital admission, and those episodes were with peak blood pressure levels of > 170/120mmHg, tachycardia 110/min, attacks of palpitation, associated headache, facial flushing and diarrhea over past 5 months.
At first cardiologist was consulted. The electrocardiogram was normal and showed sinus rhythm, the echocardiogram showed ejection fraction of 42%, compensated heart, thyroid gland with homogenic structure and beta blocker (5mg/day) and alprazolam (0.25mg/day) was prescribed by cardiologist.
A CT scan of the abdomen with contrast revealed a small focal lesion (a left-sided adrenal mass) measuring approximately 9mm by 6mm on the left adrenal gland (Figure 1). The mass appeared to have a mostly homogenous contrast enhancement. Pattern and correlation with laboratory hormonal parameters were suggested for differentiation purposes, in conjunction with control CT scans.
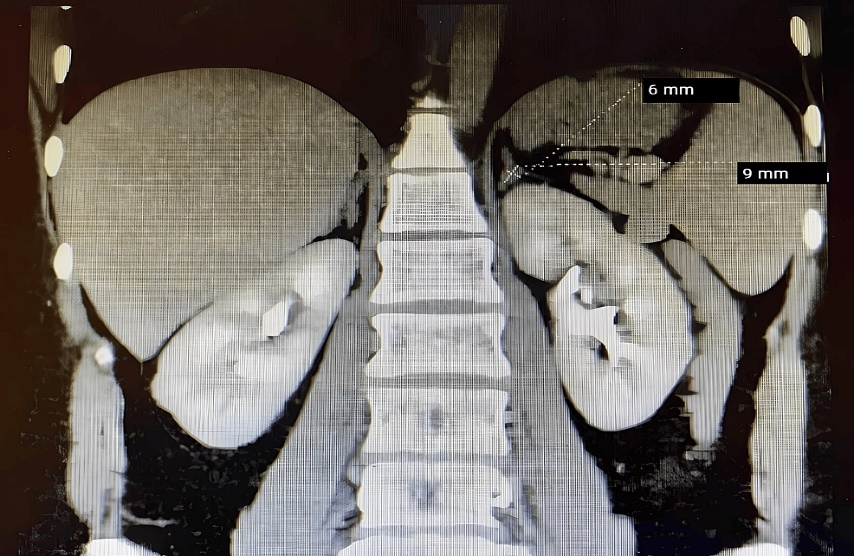
Figure 1. Abdominal CT scan showing a homogeneous lesion, measuring approximately 9mm by 6mm on the left adrenal gland.
Results
Biochemical tests were within normal ranges, except for a slightly increased CRP of 51.64mg/L, Direct Renin Immunoassay (CLIA) 56ng/l and Noradrenalin 514ng/L. Other hormonal status tests and clothing test were all normal.
A sampling of the adrenal gland was required by our urologist for the separate evaluation of the hormones of the adrenal gland, and because this method is not performed in our institution or in any other institution, this examination was performed abroad. Results showed hyperfunction on the cortex of the adrenal gland: cortisol 3.3μ/dL, aldosterone 443ng/dL and adrenalin 333pg/mL. After sampling of the gland, multidisciplinary collaboration of urologist, cardiologist, endocrinologist and anesthesiologist was made for adrenal gland laparoscopic removal due to clinical signs, and an provisional diagnosis of active adrenal gland tumor and secondary hypertension due to adrenal pheochromocytoma was confirmed by clinical signs. The patient was scheduled to undergo an excision of an adrenal mass and was referred to the anesthesiologist for evaluation.
Table 1. Biopsy results from the adrenal gland sampling.
| Sampling Results | |||
| Cortisol normal range (2.5-11.9μ/dL) | Aldosterone normal range (3.7-43.2ng/dL) | Adrenalin (1-140pg/mL) | |
| Left adrenal vein | 3.3μ/dL | 443ng/dL | 333pg/mL |
| Left peripheral vein | 1.2μ/dL, | 19.8ng/dL | 10.2pg/mL |
| Right adrenal vein | 1.6μ/dL, | 5ng/dL | 4pg/mL |
| Right peripheral vein | 1.3μ/dL, | 16.2ng/dL | 12.1pg/mL |
During the pre-op visit, the patient seemed calm, and there were no signs of pallor, cyanosis, jaundice or a rash. Preoperative standard investigations were performed. ECG, BP, systemic examination, airway assessment, biochemical laboratory and chest X-ray were all within normal ranges, and the patient was assigned ASA grade 2. Informed consent for high-risk surgery was obtained, and the patient continued taking her antihypertensive medications until the morning of the surgery.
Premedication with Diazepam 5mg was administered the night before and the morning of the surgery, and the patient fasted overnight. An intravenous saline solution of 1000mL was infused over 8 hours overnight. A preoperative preparation was performed with hydrocortisone 100mg administered the night before and the morning of the surgery according to our institutional protocol.
After the patient’s arrival in the operating room, an 18-gauge intravenous needle was inserted, and a 0.9% NaCl solution was initiated. Non-invasive devices were fixed to monitor vital signs, including automated non-invasive blood pressure, pulse oximeters and electrocardiograms. A 20-gauge needle over the right radial artery for invasive blood pressure monitoring after induction of anesthesia was placed. The patient’s baseline pulse rate was 80 beats per minute, blood pressure was 140/80mmHg and oxygen saturation was 97%.
The patient underwent general anesthesia, was pre-oxygenated with 100% oxygen, premedicated with dormicum 2mg, and induced with fentanyl 0.15μg and propofol 200mg. Muscle paralysis was achieved using rocuronium 50mg, and the patient was intubated with a 7.5-mm endotracheal tube. A nasogastric tube was inserted, and the patient was mechanically ventilated with a tidal volume of 7ml/kg at a respiratory rate per minute that was adjusted according to ETCO2, which we maintained between 35 and 42mmHg. Anesthesia was maintained using desflurane with a MAC of 6.0V% and remifentanil at 0.5mcg/kg/min. During anesthesia, the antibiotic Ceftriaxone, 2 grams and antiemetics were administered. The patient was generally stable until tumor manipulation, at which point she became hemodynamically unstable. Her blood pressure increased to 200/120mmHg, and her heart rate rose to 120 beats per minute. This was managed by increasing the depth of anesthesia and administering the short acting β blocker esmolol 30mg. After ligation of the vein and gland excision, the patient experienced hypotension of 60-40mmHg. This was corrected by replacing the volume with 1 liter of saline solution and boluses of phenylephrine at 200mcg and 0.75mcg/kg/min by intravenous continuous infusion.
The total duration of the operation was 55 minutes without significant blood loss. The patient was extubated at the end of the surgery on the operation table after receiving fentanyl 0.05mg and reversing the residual muscle paralysis with IV 1 milligrams of atropine and IV 2.5 milligrams of neostigmine.
After her surgery, she was transferred to surgical ward with adequate monitoring and postoperative therapy. Postoperative recovery was uneventful, and the patient was discharged on the third postoperative day without any complications. Surgery resulted in a significant improvement in BP control, and she showed no signs or symptoms of hypertension during a follow-up visit within 1 week of the surgery.
Discussion
Based on available literature and to our knowledge there is no single sign or symptom that can reliably diagnose or rule out pheochromocytoma. Instead, doctors can learn more from the combination of certain symptoms, findings from a physical exam and lab tests. Additional research is needed to determine the precise diagnostic value of these clinical findings. For now, the diagnosis of pheochromocytoma relies on a high degree of clinical suspicion, laboratory tests and appropriate imaging studies (4).
The clinical presentation of paroxysmal headaches, sweating, heart palpitations and hypertension, combined with abnormal results from 24-hours urine of fractionated metanephrine, and vanillylmandelic acid, also known as para- vanillylmandelic acid or P-VMA, are tests that suggest a diagnosis of pheochromocytoma, which is reinforced by the existence of an adrenal mass. Nevertheless, it is crucial to recognize that this constellation of symptoms is not universal and is observed in less than 25% of the patients diagnosed with pheochromocytomas (5).
Our presented case matches the results presented by Gerrero and colleagues in their article. They show that phaeochromocytomas can be presented differently based on factors such as tumor size and catecholamine production. It remains unclear whether the size of a pheochromocytoma is related to hormone levels, clinical presentation or complications during the perioperative period. According to their study, there is a direct relationship between the size of a tumor and the levels of hormones it produces. Tumors with smaller sizes typically have lower levels of catecholamine secretion, while larger tumors have a greater potential to secrete hormones and exhibit greater variability in their secretory capacity. Furthermore, larger tumors were found to have the highest ratios of hormone production (6).
In our case, the patient was clinically diagnosed with pheochromocytoma due to presenting typical symptoms; the adrenal mass was a very small focal lesion; and every biochemical analysis was within normal limits except the Direct Renin Immunoassay (CLIA), which was increased and associated with hyperaldosteronism (Conn’s syndrome); and noradrenaline were associated with pheochromocytoma (7). These findings were confirmed with a biopsy of the adrenal gland. Although an adrenal gland biopsy is an invasive procedure, the existing literature suggests that to help avoid potentially fatal consequences, an adrenal biopsy should only be carried out if the anticipated results are likely to change how the patient is managed specifically and after catecholamine-producing tumors have been biochemically excluded (8).
Minimally invasive surgery is the preferred option for treating this condition, with preoperative preparation including alpha-1-blockers (such as doxazosin or prazosin) and increased sodium intake for at least two weeks prior to surgery. Chronic pharmacological treatment options may include alpha1-blockers, beta-blockers (only after starting alpha1-blockers and in the presence of symptomatic tachycardia), calcium channel blockers, ACE inhibitors and central action agonists. In cases of paroxysmal hypertensive crises, emergency treatment with sodium nitroprusside or injectable phentolamine and volume replacement may be necessary. Effective treatment of neoplasms typically requires complete and timely removal to alleviate symptoms, cure hypertension, and prevent the spread of cancer cells. For individuals with malignant pheochromocytoma and metastases that cannot be surgically removed, systemic therapy with MIBG-131 is often recommended. In cases where MIBG-131 does not stop the progression of the disease or if metastases do not have MIBG uptake, cytotoxic chemotherapy may be necessary (9,10).
In our case, we administered a short-acting beta blocker before performing venous ligation on our patient, who presented with hypertension and tachycardia, and was slightly hemodynamically unstable. After the venous ligation, we administered a vasoconstrictor, phenylephrine, to stabilize the patient’s hemodynamics and maintain her blood pressure within normal limits. This approach allowed us to successfully manage the patient’s condition and ensure a safe and effective outcome. Management of perioperative hemodynamically unstable patients is presented in the literature with short-acting b blockers before ligation of the vein and vasoconstrictive therapy after removal of the gland (11).
We think that the surgeon’s speed in ligating the vein affects the patient’s intraoperative stability. The tumor size may be the reason for the normal levels of vanillylmandelic acid and metanephrine in 24-hours urine, as well as normal hormone levels, or it was due to early diagnosis and appropriate preoperative and perioperative management by the anesthesiologist, leading to a quick and successful resolution of the case without complications.
However, the most important of all is appropriate preoperative, perioperative and postoperative management with a multidisciplinary team approach, which we think is the key to success.
Conclusions
This case report highlights the importance of considering pheochromocytoma as a possible diagnosis in patients with paroxysmal hypertension, even if laboratory investigations initially appear normal. Early diagnosis, multidisciplinary collaboration within multiple specialties and adequate preoperative medical management significantly diminish morbidity and mortality during the perioperative phase among these patients. Prompt treatment with surgical excision of active adrenal gland can result in complete resolution of symptoms and prevention of complications like cardiovascular disease.
References
- Antunes E, Lopes J, Silva L, et al.: Pheochromocytoma: A Case Report. Cureus. 2022, 14:е31409. 10.7759/cureus.31409 .
- Ramachandran R, Rewari V: Current perioperative management of pheochromocytomas. Indian J Urol. 2017, 33:19-25. 10.4103/0970-1591.194781.
- Grumbach MM, Biller BM, Braunstein GD, et al.: Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med. 2003, 138:424-9. 10.7326/0003-4819-138-5-200303040-00013 .
- Soltani A, Pourian M, Davani BM: Does this patient have Pheochromocytoma? a systematic review of clinical signs and symptoms. J Diabetes Metab Disord. 2016, 15:6. 10.1186/s40200-016-0226-x .
- Westover C, Conran RM: Educational Case: Pheochromocytoma. Acad Pathol. 2018, 5:1-6. 10.1177/2374289518780500 .
- Guerrero MA, Schreinemakers JM, Vriens MR, Suh I: Hwang J, Shen WT, Gosnell J, Clark OH, Duh QY. Clinical spectrum of pheochromocytoma. J Am Coll Surg. 2009, 209:727-32. 10.1016/j.jamcollsurg.2009.09.022 .
- Pacak K, Eisenhofer G: An assessment of biochemical tests for the diagnosis of pheochromocytoma. Nat Clin Pract Endocrinol Metab. 2007, 3:744-5. 10.1038/ncpendmet0615 .
- Bancos I, Tamhane S, Shah M, et al.: Diagnosis of endocrine disease: the diagnostic performance of adrenal biopsy: a systematic review and meta-analysis. European Journal of Endocrinology. 2016, 175:65-80. 10.1530/EJE-16-0297 .
- Barroso WKS, Rodrigues CIS, Bortolotto LA, et al.: Brazilian guidelines on arterial hypertension –. 20202021, 116:516-658. 10.36660/abc.20201238 .
- Senne M, Wichmann D, Pindur P, Grasshoff C, Mueller S: Hemodynamic Instability during Surgery for Pheochromocytoma: A Retrospective Cohort Analysis. J Clin Med. 2022, 16:7471. 10.3390/jcm11247471 .
- Naranjo J, Dodd S, Martin YN: Perioperative Management of Pheochromocytoma. J Cardiothorac Vasc Anesth. 2017, 31:1427-39. 10.1053/j.jvca.2017.02.023 .