UDK: 616.24-002.7-073.756.8:004]:613.84
https://www.doi.org/10.55302/MJA2484062d
Dimitrijevikj K1, Mitreska N2, Nikolova S2
1University Clinic of Pulmonology and Allergology, Faculty of Medicine, at “Ss. Cyril and Methodius” University, Skopje, Republic of North Macedonia
2University Institute of Radiology, Faculty of Medicine, at “Ss. Cyril and Methodius University”, Skopje, Republic of North Macedonia
Abstract
Introduction: Sarcoidosis is a multisystemic granulomatous disease that usually affects lung parenchyma with interstitial and granulomatous changes of varying intensity and expression, depending on the degree of the disease.
The aim of the study: To detect pulmonary changes of sarcoidosis on high-resolution CT (HRCT) and to correlate them with smoking.
Material and Methods: Computed tomography with high resolution was made on 128 slice CT scanner PHILIPS INCISIVE, using 1mm thin-slice thickness and high spatial frequencies algorithm for image reconstruction. A total of 50 patients diagnosed with sarcoidosis who came to our University Clinic of Pulmonology and Allergology – Skopje were included in this study and their HRCT findings were compared to smokers and non-smokers.
Results: The gender structure of the patients is predominantly made up of female patients 92% vs 8% male. Reticular opacities on HRCT were more often seen in smokers compared to non-smokers, with a statistically significant difference confirmed for their peripheral and subpleural localization (p=0.0034 and p=0.0014, respectively in the upper and middle lung zones, and in the lower lung zones). Smoking patients had insignificantly more often peribronchovascular localization of reticular opacities in the upper and middle lung zones (26.67% vs 10%, p=0.28) and in the lower lung zones (26.67% vs 20%, p=0.74). Regarding the smoking status, 16% of the patients declared themselves as current smokers, 56% as ex-smokers, with an average smoking experience of 14.9 ± 4.8 years.
Conclusion: HRCT is the method of choice in the evaluation of pathological changes in pulmonary sarcoidosis. It shows very precisely the characteristic findings of lymph nodes, micronodules and other lesions, their distribution, as well as atypical changes. Smoking plays a certain role in the interstitial changes of the patients with sarcoidosis, although we do not have data whether smoking has effects on the extent, course or outcome of the disease.
Keywords: high-resolution computer tomography; interstitial disease; nonsmokers; sarcoidosis; smoking.
Introduction
Sarcoidosis is a multisystemic granulomatous disease that usually affects the lung parenchyma with interstitial and granulomatous changes of varying intensity and expression, depending on the degree of the disease. Although many hypotheses have been proposed, the etiology of sarcoidosis remains unclear (1). Very often, hilar and mediastinal lymphadenopathy with changes of various types is seen, but peripheral groups of lymph nodes can also be involved, such as axillary, inguinal and cervical (1-3).
Sarcoidosis mainly affects adults under 40 years of age (with a peak in the third decade of life) and a second peak occurs in women over 50 years of age in countries such as Japan (4), but it can also occur at a younger age. The disease is diffusely distributed throughout the world and affects both genders almost equally with a slight predominance among women (3,5). The prevalence of the disease is higher among non-smokers than among smokers, and is more common in certain occupational groups, such as nurses, firefighters, transport and service workers, although the cause is unknown (5).
Imaging methods such as high-resolution computed tomography (HRCT) play key role in the diagnosis and monitoring of the patients. This is due to the fact that the plain radiograph of the lungs has many limitations, including insufficient resolution in the detection of pulmonary abnormalities and in the detection of hilar and mediastinal adenopathy.
HRCT has become a powerful tool and has greater superiority than conventional computed tomography in the detection and evaluation of subtle parenchymal lesions and abnormalities of lung structures (6). It helps us in the prognostic course and development of the disease, identification of irreversible changes, potentially reversible and the appropriate treatment.
In advanced stages of sarcoidosis, reticular opacities zones, tractional bronchiectasis, architectural distortion of the parenchyma, honeycomb lung, bullae, and paracicatricial emphysema are seen in the upper and middle lung zones. The lung bases are usually spared (7). On HRCT, 75–95% of the patients have mediastinal and hilar lymphadenopathy, and approximately 67–75% have hilar lymphadenopathy, usually bilateral, but not infrequently with a right-sided predominance (8,9). Lymph nodes are more often focally calcified than completely and dense, amorphous or nebulous, rod-like or eggshell-like in 3% of the patients within 5 years of diagnosis of the disease, or in 20% after 10 years (10).
Sarcoid nodules formed in the lung parenchyma are seen in nearly half of the patients, and in some studies up to over 90% of the patients (9, 11). They are typically predominant in the upper lobes but may also be seen with more diffuse distribution. The lower lobes are usually spared. Micronodules have dimensions below 1cm, and HRCT can detect changes up to 1-2mm in diameter. The presence of small, 1-5mm nodules clearly demarcated, or with irregular contours, is the most common and almost universal finding seen in the lung (12).
Although nodules appear as predominant finding in sarcoidosis, the parenchymal form of the disease includes opacities of different sizes (1-10cm) ground glass type, consolidation, reticulation. They predominate in the peripheral middle zones, while the costophrenic angles are spared.
Small nodules in sarcoidosis have a characteristic perilymphatic and symmetrical distribution. That distribution corresponds to nodules identified in the peribronchovascular interstitium, interlobar septa, because the lymphatic tract lies along these two anatomical structures of the lungs. The subpleural zone, as well as the large and small fissures that are an extension of the pleura and belong to the lymphatic system are also often involved (13,14).
HRCT findings of fibrosis include reticulations, architectural distortion, tractional bronchiectasis and volume loss primarily in the upper or central lung. As fibrosis progresses, larger zones of mass-like consolidation occur in the perihilar regions.
Honeycomb lungs and lung cysts may also occur predominantly in the upper and middle lung lobes, in contrast to the basally predominant fibrosis and honeycombing in UIP and IPF.
Sarcoidosis mainly affects non-smokers, but in this article we will corelate the disease with smokers and past smokers and their HRCT changes, because in our country the dominant population is a smoker (15).
The aim of the study is to detect pulmonary changes of sarcoidosis on HRCT and to correlate them with smoking.
Material and Methods
Patients voluntarily participated in the study with a previously signed informed consent. The study was conducted with the consent of the Ethics Commission of the Faculty of Medicine in Skopje and was in accordance with the ethical principles of the Helsinki Declaration of the World Medical Association for medical research involving human subjects.
A total of 50 patients diagnosed with sarcoidosis came to our University Clinic of Pulmonology and Allergology-Skopje. HRCT was performed on a 128-slice PHILIPS INCISIVE computed tomography scanner, using 1mm thickness of sections and high spatial frequencies algorithm for image reconstruction.
The images were evaluated using appropriate lungs and mediastinal windows. Lymph nodes were classified as hilar and mediastinal with maximum short axis diameter (MSAD), more than 10mm taken as their enlargement.
Pulmonary changes (opacity) were classified as nodules (micronodules 1-3mm and macronodules greater than 5mm), reticular opacities, fibrous lesions, ground glass opacities and confluent consolidations. Nodular distribution was classified as perilymphatic, centrilobular and randomized. The predominant distribution of lesions in different zones of the lungs (upper, middle and lower zones) was also noted.
Results
This statistical analysis includes 50 patients diagnosed with sarcoidosis. The gender structure of the patients is predominantly made up of female patients – 46 (92%) vs 4 (8%) male.
The patients were aged from 30 to 73 years, with an average age of 52.6 ± 12.5 years, and with a place of residence mostly in an urban environment – 42 (84%) vs 8 (16%).
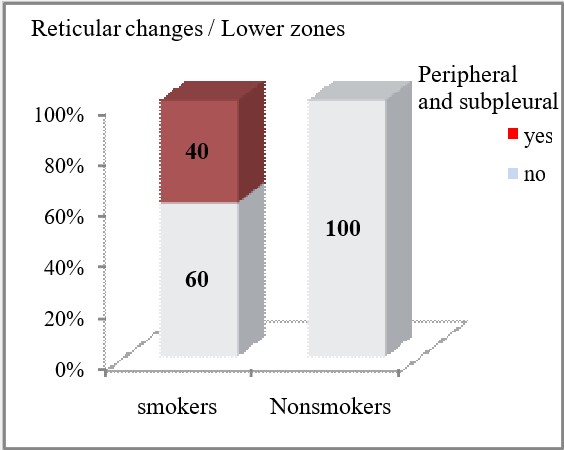
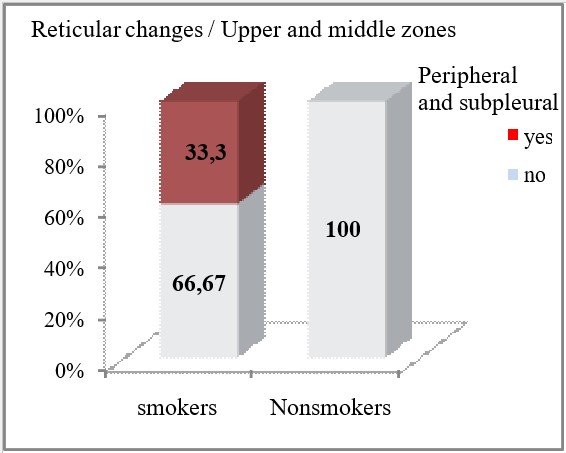
Reticular opacities on HRCT were more often seen in smokers compared to non-smokers, with a statistically significant difference confirmed for their peripheral and subpleural localization (p=0.0034 and p=0.0014, respectively in the upper and middle lung zones, and in the lower lung zones). Smoking patients had insignificantly more often peribronchovascular localization of reticular opacities in the upper and middle lung zones (26.67% vs 10%, p=0.28) and in the lower lung zones (26.67% vs 20%, p=0.74).
Regarding the smoking status, 8 (16%) patients declared themselves as current smokers, 28 (56%) as ex-smokers, with an average smoking experience of 14.9 ± 4.8 years. The time when they stopped smoking ranges between 4 and 27 years, on average ex-smokers stopped smoking 11.9 ± 7.1 years ago.
Due to lung disease, 16 (32%) patients were hospitalized, all with one hospitalization.
2 (4%) patients had a positive family history of lung disease.
Table 1. Patients’ characteristics.
| Variable | n (%) |
| Gender
female male |
46 (92) 4 (8) |
| Age
mean ± SD (min- max) |
52.6 ± 12.5 (30 – 73) |
| Place of living
town village |
42 (84) 8 (16) |
| Do you smoke
yes no |
8 (16) 42 (84) |
| Have you ever smoked
yes no |
28 (56) 22 (44) |
| How many years have you smoked
(mean ± SD) (min- max) |
14.9 ± 4.8 (7 – 20) |
| How many years ago did you stop smoking
(mean ± SD) (min- max) |
11.9 ± 7.1 (4 – 27) |
Table 2. Distribution of reticular opacities depending on smoking status.
| HRCT findings | Smokers | p-level | |||
| yes
n (%) |
no
n (%) |
||||
| Reticular changes | |||||
| Upper and middle zones | Peripheral and subpleural | yes | 10 (33.33) | 0 | **p=0.0034 |
| no | 20 (66.67) | 20 (100) | |||
| Peribronchovascular | yes | 8 (26.67) | 2 (10) | p=0.28 | |
| no | 22 (73.33) | 18 (90) | |||
| Lower zones | Peripheral and subpleural | yes | 12 (40) | 0 | **p=0.0014 |
| no | 18 (60) | 20 (100) | |||
| Peribronchovascular | yes | 8 (26.67) | 4 (20) | p=0.74 | |
| no | 22 (73.33) | 16 (80) | |||
p (Fisher’s exact test)
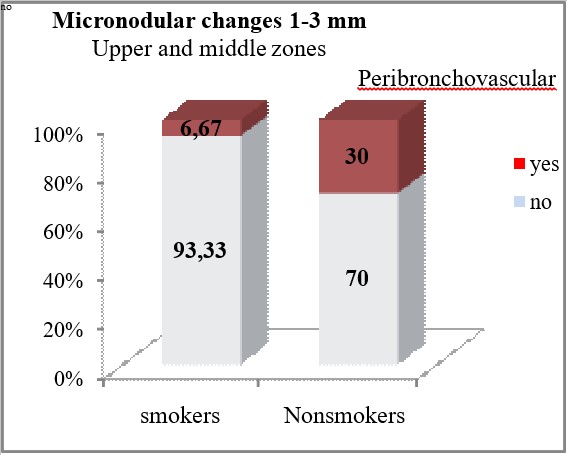
Micronodular opacities with a size of 1 to 3mm localized peribronchovascular in the upper and middle zones were significantly less frequent HRCT findings in smokers compared to non-smokers (6.67% vs 30%, p=0.047). Perilymphatic micronodular opacities with size of 1 to 3mm were diagnosed insignificantly more often in smokers, in the peribronchovascular regions of the upper and middle zones (33.33% vs 20%, p=0.353), in the subpleural regions of the upper and middle zones (26.67% vs 10%, p=0.28), in the peribronchovascular regions of the lower zones (6.67% vs 0%, p=0.51), and in the subpleural regions of the lower zones (6.67% vs 0%, p=0.51).
Table 3. Distribution of micronodular changes from 1-3mm depending on smoking status.
| HRCT findings | Smokers | p-level | |||
| yes
n (%) |
no
n (%) |
||||
| Micronodular changes 1-3mm | |||||
| Upper and middle zones | Centrolobular | yes | 0 | 0 | |
| no | 30 (100) | 20 (100) | |||
| Peribronchovascular | yes | 2 (6.67) | 6 (30) | *p=0.047 | |
| no | 28 (93.33) | 14 (70) | |||
| Lower zones | Centrolobular | yes | 0 | 0 | |
| no | 30 (100) | 20 (100) | |||
| Peribronchovascular | yes | 2 (6.67) | 2 (10) | p=1.0 | |
| no | 28 (93.33) | 18 (90) | |||
| Micronodular changes (1-3mm) perilymphatic | |||||
| Upper and middle zones | Peribronchovascular | yes | 10 (33.33) | 4 (20) | p=0.35 |
| no | 20 (66.67) | 16 (80) | |||
| Subpleural | yes | 8 (26.67) | 2 (10) | p=0.28 | |
| no | 22 (73.33) | 18 (90) | |||
| Lower zones | Peribronchovascular | yes | 2 (6.67) | 0 | p=0.51 |
| no | 28 (93.33) | 20 (100) | |||
| Subpleural | yes | 2 (6.67) | 0 | p=0.51 | |
| no | 28 (93.33) | 20 (100) | |||
p (Fisher’s exact test)
*Significance p<0.05
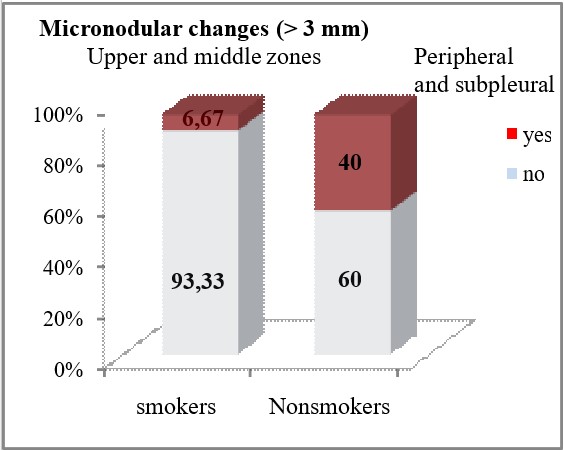
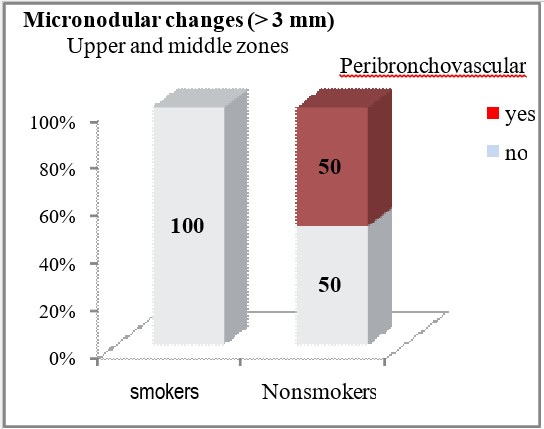
HRCT finding of micronodular changes greater than 3mm in the upper and middle lung zones was significantly associated with the smoking status of the patients (p<0.05). In non-smokers, micronodular changes greater than 3mm peripherally and subpleural, were significantly less often seen on HRCT (6.67% vs 40%, p=0.0088), while peribronchovascular localization of these changes in the upper and middle lung zones was seen only in 50% of the patients from the group of non-smokers (p=0.00002). Micronodular changes larger than 3mm in the lower lung zones were registered only in non-smokers (10%), but no statistically significant difference was confirmed between smokers and non-smokers in terms of the frequency of finding micronodular shadows larger than 3mm in the lower lung zones (p=0.155).
Table 4. Distribution of micronodular changes larger than 3mm depending on smoking status.
| HRCT findings | Smokers | p-level | |||
| yes
n (%) |
no
n (%) |
||||
| Micronodular changes (>3mm) | |||||
| Upper and middle zones | Peripheral and subpleural | yes | 2 (6.67) | 8 (40) | **p=0.0088 |
| no | 28 (93.33) | 12 (60) | |||
| Peribronchovascular | yes | 0 | 10 (50) | ***p=0.00002 | |
| no | 30 (100) | 10 (50) | |||
| Lower zones | Peripheral and subpleural | yes | 0 | 2 (10) | p=0.155 |
| no | 30 (100) | 18 (90) | |||
| Peribronchovascular | yes | 0 | 2 (10) | p=0.155 | |
| no | 30 (100) | 18 (90) | |||
p (Fisherꞌs exact test)
**sig p<0.01, ***sig p<0.0001
GG opacities in the peripheral and subpleural regions of the lungs were detected only in smoking patients (6.67%), in 20% of the smokers in the central regions of the upper and middle zones, in 33.33% of the smokers and in 10% of the non-smokers in the central regions of the lower zones, without statistically significant difference in all localizations (p>0.05).
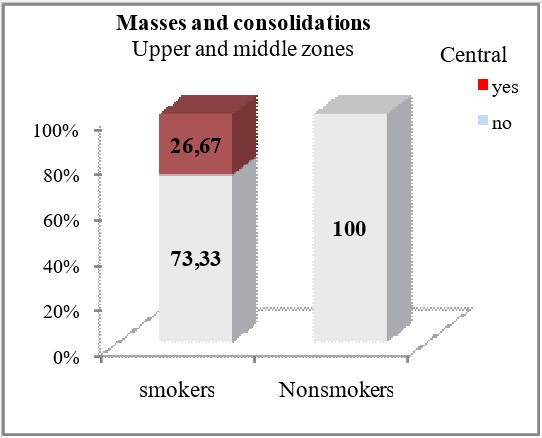
Masses and consolidations centrally localized in the upper and middle lung zones were observed only in smokers (26.67%), with a significant difference for p=0.015. Smokers and non-smokers did not differ significantly in terms of HRCT findings of hypoattenuation type changes (p>0.05). Smokers had insignificantly more often such changes in the upper and middle lung zones (13.33% vs 10%) in the peripheral and subpleural regions, (20% vs 10%) in the central regions.
Table 5. Distribution of GG opacities, masses, consolidations and hypoattenuation depending on smoking status.
| HRCT findings | Smokers | p-level | |||
| yes
n (%) |
no
n (%) |
||||
| GG Opacities | |||||
| Upper and middle zones | Peripheral and subpleural | yes | 2 (6.67) | 0 | p=0.51 |
| no | 28 (93.33) | 20 (100) | |||
| Central | yes | 6 (20) | 0 | p=0.07 | |
| no | 24 (80) | 20 (100) | |||
| Lower zones | Peripheral and subpleural | yes | 2 (6.67) | 0 | p=0.51 |
| no | 28 (93.33) | 20 (100) | |||
| Central | yes | 10 (33.33) | 2 (10) | p=0.09 | |
| no | 20 (66.67) | 18 (90) | |||
| Masses and consolidations | |||||
| Upper and middle zones | Peripheral and subpleural | yes | 0 | 2 (10) | p=0.155 |
| no | 30 (100) | 18 (90) | |||
| Central | yes | 8 (26.67) | 0 | *p=0.015 | |
| no | 22 (73.33) | 20 (100) | |||
| Lower zones | Peripheral and subpleural | yes | 4 (13.33) | 4 (20) | p=0.7 |
| no | 26 (86.67) | 16 (80) | |||
| Central | yes | 0 | 0 | ||
| no | 30 (100) | 20 (100) | |||
| Hypoattenuation | |||||
| Upper and middle zones | Peripheral and subpleural | yes | 4 (13.33) | 2 (10) | p=1.0 |
| no | 26 (86.67) | 18 (90) | |||
| Central | yes | 6 (20) | 2 (10) | p=0.45 | |
| no | 24 (80) | 18 (90) | |||
| Lower zones | Peripheral and subpleural | yes | 4 (13.33) | 4 (20) | p=0.7 |
| no | 26 (86.67) | 16 (80) | |||
| Central | yes | 0 | 2 (10) | p=0.155 | |
| no | 30 (100) | 18 (90) | |||
p (Fisher’s exact test)
According to the obtained results, lymphadenopathy was not presented as a significantly different HRCT finding in smokers and non-smokers (p>0.05). Bilateral hilar lymphadenopathy (66.67% of smokers and 70% of non-smokers) and right paratracheal lymphadenopathy (73.33% of smokers and 90% non-smokers) were predominant in both groups.
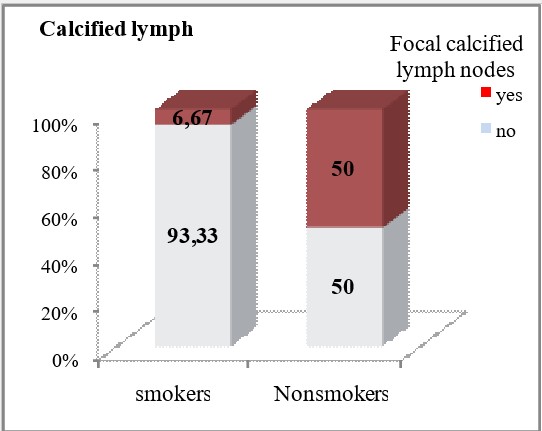
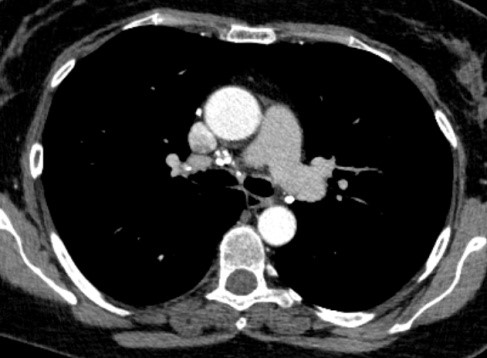
Focal large calcifications were significantly less frequently detected in smokers (6.67% vs 50%, p=0.0007).
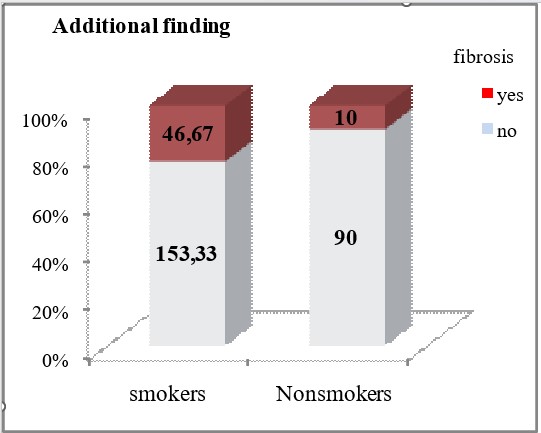
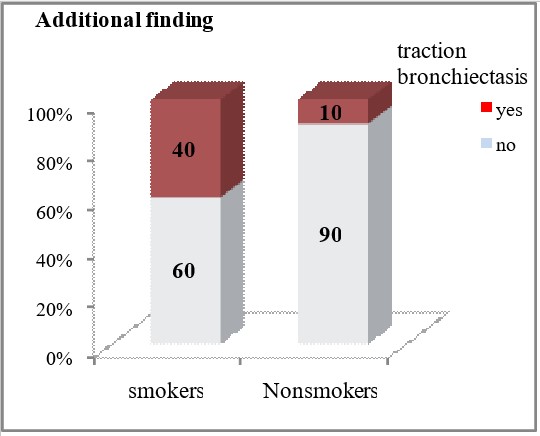
Smokers had significantly more frequent HRCT findings of traction bronchiectasis (40% vs 10%, p=0.026) and fibrosis (46.67% vs 10%, p=0.012).
Only 6.67% of the smoking patients had a finding of honeycomb lung, but without statistically confirmed association with this group of patients (p=0.51).
Table 6. Distribution of lymphadenopathy, calcified lymph nodes and additional findings depending on smoking status.
| Variable | Smokers | p-level | |||
| yes
n (%) |
no
n (%) |
||||
| Lymphadenopathy | Bilateral hilar | yes | 20 (66.67) | 14 (70) | p=1.0 |
| no | 10 (33.33) | 6 (30) | |||
| Right paratracheal | yes | 22 (73.33) | 18 (90) | p=0.28 | |
| no | 8 (26.67) | 2 (10) | |||
| Others lymph nodes stations | yes | 20 (66.67) | 16 (80) | p=0.35 | |
| no | 10 (33.33) | 4 (20) | |||
| Conglomerate lymph nodes | yes | 4 (13.33) | 6 (30) | p=0.17 | |
| no | 26 (86.67) | 14 (70) | |||
| Calcified lymph nodes | Focal calcified lymph nodes | yes | 2 (6.67) | 10 (50) | ***p=0.0007 |
| no | 28 (93.33) | 10 (50) | |||
| Punctiform | yes | 0 | 2 (10) | p=0.155 | |
| no | 30 (100) | 18 (90) | |||
| Egg shell | yes | 2 (6.67) | 0 | p=0.51 | |
| no | 28 (93.33) | 20 (100) | |||
| Additional finding | Bullae | yes | 8 (26.67) | 4 (20) | p=0.74 |
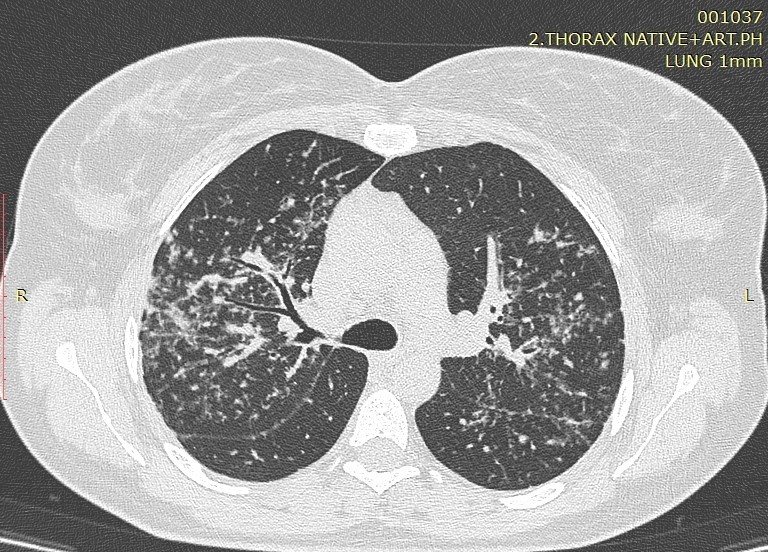
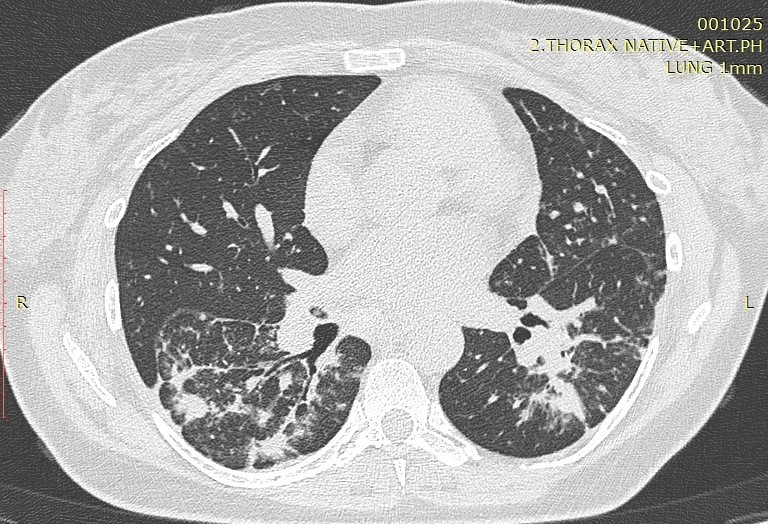
Image1. Perilymphatic distribution of sarcoidosis
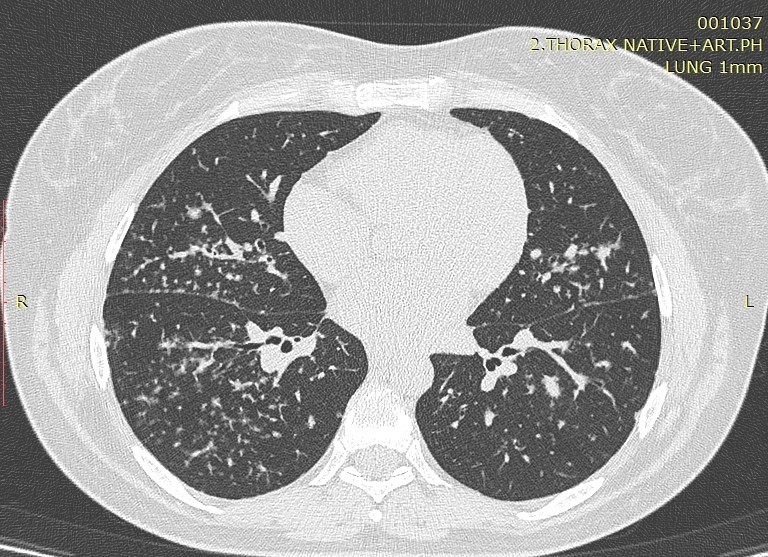
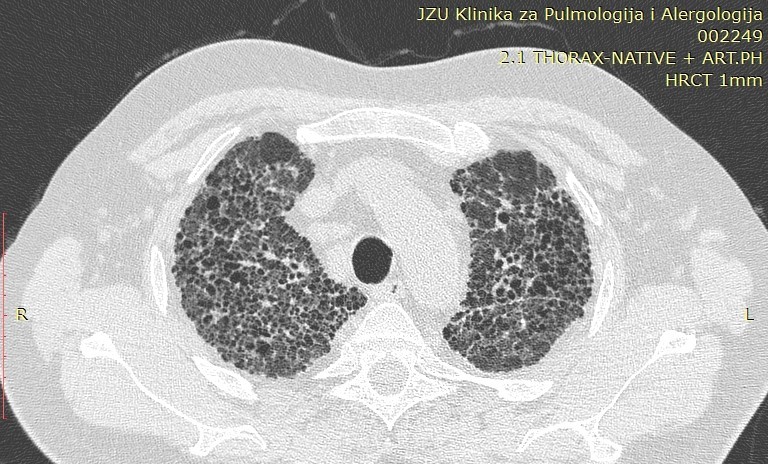
Image 2. Typical perilymphatic distribution of sarcoidosis
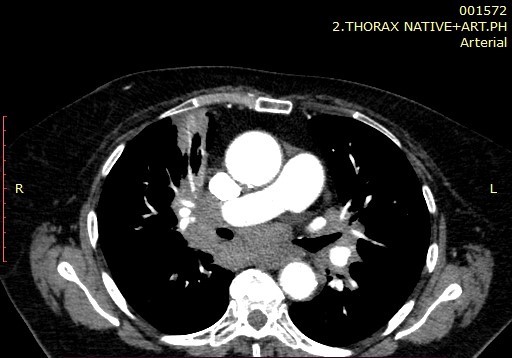
Image 3. Mediastinal Lymphadenopathy.
Image 4. Calcified lymph nodes.
Image 5. Atypical manifestation of sarcoidosis.
Image 6. Atypical manifestation of sarcoidosis.
Image 7. End stage sarcoidosis-fibrosis.
Discussion
Sarcoidosis, with a total number of 50 patients analyzed in our study, is a multisystemic granulomatous disease of unknown etiology. Pulmonary involvement is the most common presentation of the disease with interstitial and granulomatous changes of different location, intensity and expression, depending on the stage of the disease. The staging of the disease is still done according to the established radiological criteria of the conventional radiogram of the lungs, despite the great sensitivity of HRCT in detecting small changes in the lungs that are not visible on the ordinary radiogram. According to Lynch, the plain radiogram detects only 50-60% of the lymph nodes and 30-40% of the parenchymal abnormalities found with HRCT. Only in few cases, patients with transbronchial biopsy-confirmed sarcoidosis had a normal HRCT scan (16). Involvement of the hilar and mediastinal lymph nodes is seen in 50-90% of the patients, usually bilaterally. The disease affects both genders with a slight predominance in women, in whom a second peak can be seen after the age of 50 (Japan). Despite the fact that etiology is unknown, certain professions are still observed to increase the predisposition to this disease. These are nurses, hygienists, administrators in the chemical industry, dispatchers, firefighters. It mainly occurs in non-smokers with predominant symptoms of dry cough and dyspnea. Perilymphatic distribution of pulmonary granulomas is regularly detected by HRCT. A study by Herreaz from 2010 notes that the most common characteristic finding is small clearly circumscribed nodules between 2 and 5mm in size and with a lymphangitic distribution. Although these lesions are seen in the central lung zones, usually with a peribronchovascular and centrilobular distribution, they are more commonly seen in the peripheral lung, usually with a centrilobular and subpleural distribution along the fissures. The involvement is usually symmetrical, and the affected zones are mostly upper and middle lung parts (16). The perilymphatic distribution of micronodules, the interstitial changes as well as the additional (atypical) finding of sarcoidosis are analyzed in this article.
In this study, the patients are mainly female, i.e. 46 (92%) versus male with 4 (8%), aged 30-73 years, mostly living in an urban environment. Regarding the smoking status, 8 (16%) patients declared themselves as current smokers and 42 (84%) as non-smokers, but 28 (56%) of them were smokers in the past. The article compares the distribution of reticular shadows that were more often seen in smokers compared to non-smokers, with a statistically significant difference confirmed for their peripheral and subpleural localization (for p=0.0034 and p=0.0014), respectively in the upper and middle lung zones and in the lower lung zones.
HRCT findings of reticular opacities were not significantly associated with cough (p> 0.05).
Micronodular lung changes detected by HRCT and 1-3mm in size were more frequent compared to those of 3mm – 40 (80%) vs 24 (48%). Micronodules 1-3mm predominated in the upper and middle zones 32 (64%), while in the lower zones there were 8 (16%) patients. A statistical analysis and comparison of this type of micronodules in smokers and non-smokers was also made, where it was concluded that micronodular opacities with a size of 1-3mm localized peribronchovascular in the upper and middle lung zones were significantly less HRCT findings in smokers compared to non-smokers (6.67% vs 30%, p=0.047). Micronodules over 3mm also predominated according to localization in the upper and middle zones with 20 (40%) patients compared to the lower zones with 4 (8%) patients. HRCT findings of micronodular changes larger than 3mm in the upper and middle lung zones were significantly associated with the smoking status of the patients. In non-smokers, micronodular changes greater than 3mm peripherally and subpleural were seen significantly less often (6.67% vs 40%, p=0.0088), while the peribronchovascular localization of these changes in the upper and middle pleural zones was seen only in 50% of the patients from the non-smoking group (p=0.00002). The finding of GG opacities dominates in the lower zones, that is, in 14 (28%) of the patients compared to 8 (16%) patients who had this finding in the upper and middle lung zones.
Masses and consolidations easily predominated in the upper and middle zones with 10 (20%) vs 8 (16%) in the lower zones. Also, hypoattenuating lesions easily predominate in the upper and middle zones, compared to the lower, 11 (28%) versus 10 (20%). Regarding additional findings such as GG opacities, masses and consolidations and hypoattenuation-type changes, a significant difference for p=0.015 was observed in masses and consolidations centrally localized in the upper and middle lung zones only in smokers (26.67%). The stage of the disease had no significant effect on the frequency of findings of masses and consolidations (p> 0.05). Bilateral lymphadenopathy by HRCT was diagnosed in 34 (68%) patients, right paratracheal lymphadenopathy in 40 (80%), in the remaining nodal stations in 36 (72%) patients, and in 10 (20%) conglomerated lymph nodes were seen. Calcified lymph nodes were presented in 16 (32%) patients, out of which with focal large calcifications in 12 (24%) patients, punctiform in 2 (4%) and scaly also in 2 (4%) patients. The type of calcifications did not show significance in relation to the stage of the disease. Regarding the smoking status, according to the obtained results, lymphadenopathy was not presented as a significantly different HRCT finding in smokers and non-smokers (p> 0.05).
Focal large calcifications were significantly less frequently detected in smokers (6.67% vs 50%, p = 0.0007). Smokers have significantly more frequent HRCT findings of traction bronchiectasis (40% vs 10%, p=0.026) and fibrosis (46.67% vs 10%, p=0.012).
It is worth highlighting in our study that a correlation was made between the findings and the distribution of the reticular opacities according to the zones and the smoking status. In general, sarcoidosis is not associated with smoking, but since smoking causes an interstitial reaction, and there are a relatively large number of smokers and ex-smokers in the group, our goal was to see if there was significance in that regard. Reticular opacities on HRCT in sarcoidosis were more often seen in smokers compared to non-smokers with statistical significance. We confirm a difference for their presence in the upper middle as well as lower peripheral and subpleural zones. Reticular changes were present in 33.3% of smokers in the upper and middle zones, peripherally and subpleural in contrast to non-smokers where they were absent. While in the lower zones, reticular opacities were present in 40% of the smokers.
Conclusion
HRCT thin-section imaging capabilities and high spatial resolution to generate high-quality images result in better characterization and determination of abnormal changes in the lung parenchyma and interstitium. HRCT is superior to conventional CT for showing subtle parenchymal lesions and aids in the differentiation of active lesions from terminal changes. Pulmonary sarcoidosis shows spontaneous remission in approximately half of the cases within the first two years and an additional number within 5 years. However, it is estimated that for 20% of the patients it is progressive and chronic with the development of pulmonary fibrosis and significant functional insufficiency (3, 17), which leads to 5% mortality, which is why timely and accurate diagnosis is necessary. HRCT is the method of choice in the evaluation of pathological changes in pulmonary sarcoidosis, a disease that shows a wide range of radiological manifestations and is a challenge for radiologists. It shows very precisely the characteristic findings of lymph nodes, micronodules and other lesions, their distribution, as well as atypical changes.
It helps us to guide the therapy accordingly by differentiating active lesions from irreversible fibrosis, and knowing the key and characteristic signs of the disease in parallel with the clinical symptomatology ensures that the radiologist can make a specific diagnosis.
Based on the results of this study we conclude that smoking plays a certain role in the interstitial changes of the patients with sarcoidosis detected on HRCT, although we do not have data whether smoking has effects on the extent, course or outcome of the disease.
References:
- Statement of sarcoidosis: Joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of sarcoidosis and Other Granulomatous Disorders(WASOG) adopted by theATS board of directors and by the ERS executive committee, February 1999. Am J Crit Care Med 1999; 160;736-755.
- Dhagat PK, Singh S, Jain M, Singh SN, Sharma RK.Thoracic Sarcoidosis: Imaging with High Resolution Computed Tomography.J Clin of Diagn Res.2017; 11(2):TC15-TC18. https://www.doi.org/10.7860/JCDR/2017/24165/9459.
- KIM JE, Callard RH, King ET JR. Rheumatoid Artritis- Associated Interstitial Lung Disease. CHEST 2009; 136:1397–1405. DOI: 10.1378/chest.09-0444.
- Muller-Quernheim J, Prasse A, Zissel G: Sarcoidosis, Presse Med. 2012 Jun ;41(6 Pt 2): e275-87. doi: 10.1016/j.lpm.2012.03.018. Epub 2012 May 15.
- Criado E, Shanchez M, Ramirez J, Arguis P et al. Pulmonary Sarcoidosis: Manifestationof High Resolution CT with Pathologic Correlation, Chest Imaging, 2010; 1567- 1569.
- Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med 2018;6 (2):138-153.
- Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years observation. Br Med J. 1961; 2:1165-72.
- Patil SN, Levin DL: Distribution of thoracic lymphadenopathy in sarcoidosis using computed tomography. J Thorac Imaging 1999; 14: 114-117.
- Ors F, Gumus S, aydogan M, et al: HRCT findings of pulmonary sarcoidosis; relation to pulmonary function tests. Multidiscip Respir Med 2013; 8:1-8.
- Craido E, Sanches M, Ramirez J et al. Pulmonary Sarcoidosis; typical and atypical manifestations at high-resolution CT with pathologic correlation. RadioGraphics 2010; 30(6):1567-1586.
- Nishino M, Lee KS, Itoh H, et al: The spectrum of pulmonary sarcoidosis: Variations of high- resolution CT findingsand clues for specific diagnosis. Eur J Radiol 2010; 73:66-73.
- Wilson AG, Hansel DM. immunologic diseases of the lung. In: Armstrong P, Wilson AG, Dee P, Hansell DM, editors. Imaging of the diseases of the chest.3rd ed. NW, London: Mosby; 2000.p. 637-88.
- Webb WR, Muller NL, Naidich DP. High-resolution CT of the lung, 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2000.
- ItohH, Nishino M, Hatabu H. Architecture of the lung: morphology and function. J Thorac Imaging 2004; 19:221-7.
- Rosen Y: Pathology of sarcoidosis. Semin Respir Crit Care Med 2007; 28:36-52.
- Wuyts WA et all. Differential diagnosis of ususal interstitial pneumonia: when is it truly idiopathic? European Respiratory Reviev 2014; 23: 308-319.
- Martin SG, Kronek LP, Valeyre D, Brauner N, Brillet PY, Nunes H, et al. High-resolutioncomputed tomography to differentiate chronic diffuse interstitial lung diseases with predominant ground-glass pattern using logical analysis of data.eur Radiol.2010;20:1297- 1310.