UDK: 616-006.441-085.277-073.763.5
Mihajlovska Michevska T., Bundovska Kocev S., Hadji Nikolova N., Gjorgjioska S., Edis Jukic, D. Belicevska Stavrevska.
University Institute of Radiology, Faculty of Medicine, “Ss. Cyril and Metodhodius” University, Skopje, Republic of North Macedonia
Abstract
Burkitt lymphoma, classified as a subtype of non-Hodgkin lymphoma, primarily targets children. It frequently presents with extranodal involvement, often manifesting as an abdominal or pelvic mass upon initial presentation. Treatment regimen commonly incorporates chemotherapy, wherein the prognosis, particularly in pediatric cases, is notably favorable, with survival rates exceeding 90%. However, the utilization of methotrexate, a chemotherapy agent employed in hematological malignancies and other neoplasms, warrants careful consideration due to its propensity for neurotoxicity. Methotrexate-induced neurotoxicity may be presented across a spectrum of acute and chronic leukoencephalopathies. One significant manifestation is toxic encephalopathy, characterized by its predominant affliction of subcortical white matter. Additionally, notable findings include confluent hyperintensities observable on T2 and FLAIR imaging, particularly evident in the centrum semiovale region. Magnetic resonance imaging (MRI) stands as the preferred diagnostic modality. This non-invasive and sophisticated imaging technique holds immense clinical and research utility. Leveraging MRI enables early detection of neurological conditions, facilitates ongoing monitoring of treatment outcomes, and supports timely interventions, thereby offering significant benefits in patient’s care.
We report the case of a 4-years-old patient diagnosed with Burkitt lymphoma, who underwent an MRI scan following symptoms of blindness and convulsions. Initial CT scans showed no discernible pathology. Subsequent MRI findings revealed signal abnormalities, typical of methotrexate-related leukoencephalopathy. Notably, convulsions ensued shortly after initiating methotrexate treatment. These MRI findings are characteristic of toxic encephalopathy, highlighting the importance of vigilant monitoring in patients undergoing methotrexate therapy.
Key Words: MRI, Burkitt lymphoma, Methotrexate leukoencephalopathy, toxic leukoencephalopathy.
Introduction
Burkitt lymphoma is recognized for its aggressive nature and can be manifested in various anatomical locations, including the head and neck, pleural space, gastrointestinal tract, retroperitoneum, peritoneum, kidneys and gonads. The radiographic features of Burkitt lymphoma vary depending on the organ involved. Abdominal presentations may or may not include a palpable mass. In our case, a palpable abdominal mass led to a CT-guided biopsy, confirming the diagnosis of Burkitt lymphoma via histopathology.
Upon hospitalization, the prescribed therapy involved the administration of methotrexate. However, shortly after initiating methotrexate treatment, the patient exhibited symptoms including convulsions. Methotrexate, a potent anticancer drug, can induce neurotoxicity, particularly in high doses. Its mechanism of action involves competitive inhibition of dihydrofolate reductase, thereby depleting DNA precursors. The exact mechanism underlying methotrexate-induced leukoencephalopathy remains unclear, though it is proposed to involve the release of adenosine, resulting in cerebral blood vessel dilation, neuronal dysfunction and cytotoxic edema.
Methotrexate can be administered orally, intravenously or intrathecally. MRI serves as the preferred diagnostic imaging modality, with neurotoxic side effects, such as leukoencephalopathy, manifesting as transient hyperintense regions on T2-weighted imaging (T2WI), diffusion restriction, and abnormal or absent contrast enhancement (1, 2, 3). Sequential MRI examinations enable the detection of leukoencephalopathy in symptomatic patients and may reveal abnormal findings in asymptomatic individuals following methotrexate treatment (4).
In some cases, discontinuation of methotrexate therapy may be necessary, as the brain abnormalities associated with the acute toxic leukoencephalopathy can potentially reverse with therapy or removal of the offending agent during the early phase of onset.
Case Report
We present an intriguing case involving a four-years-old child exhibiting abdominal manifestations of Burkitt lymphoma alongside methotrexate-related leukoencephalopathy. The patient’s initial presentation occurred in April 2024, when she was presented to the emergency department with a distended abdomen persisting for one week, accompanied by the presence of a palpable solid mass in the abdominal region. Subsequently, the patient underwent a CT scan of the abdomen with intravenous contrast for further evaluation.
CT Findings
The CT scan showed a large, expanding mass in the abdominal cavity, involving the mesentery and displacing bowel loops without causing obstruction. The mass extended from below the pancreas to the suprapubic region and surrounded the uterus, making the ovaries indistinguishable. There were also enlarged lymph nodes near the aorta and below the diaphragm. The findings suggested a potentially aggressive process requiring further investigation and multidisciplinary management. The patient, diagnosed with Burkitt lymphoma through clinical, biochemical and molecular assessments by the pediatric hemato-oncology team, was hospitalized.
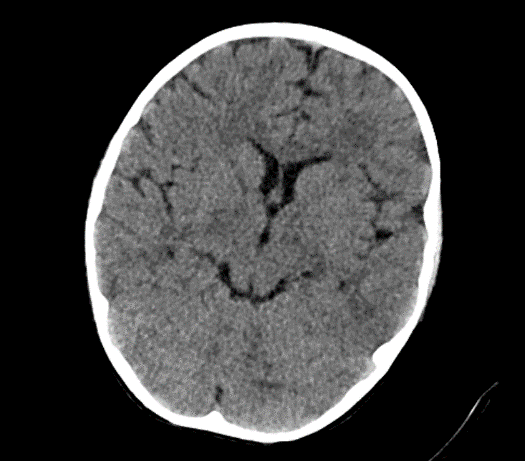
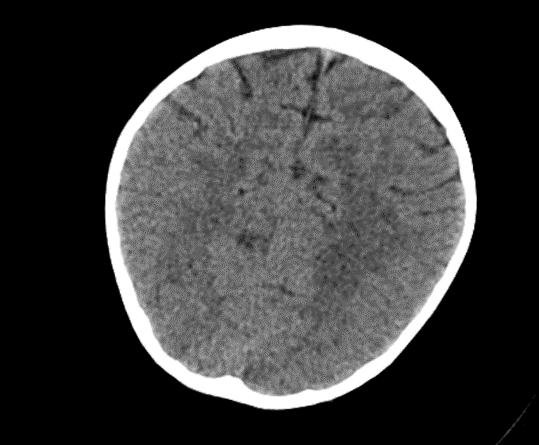
Due to the exacerbation of symptoms and onset of convulsions, a brain CT was conducted, which revealed no significant abnormalities.
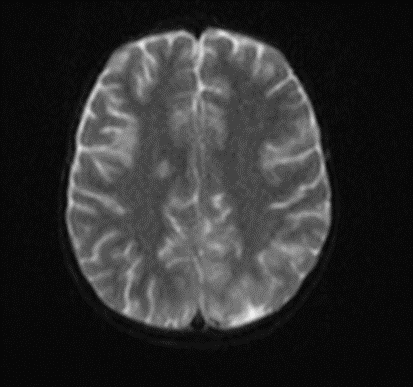
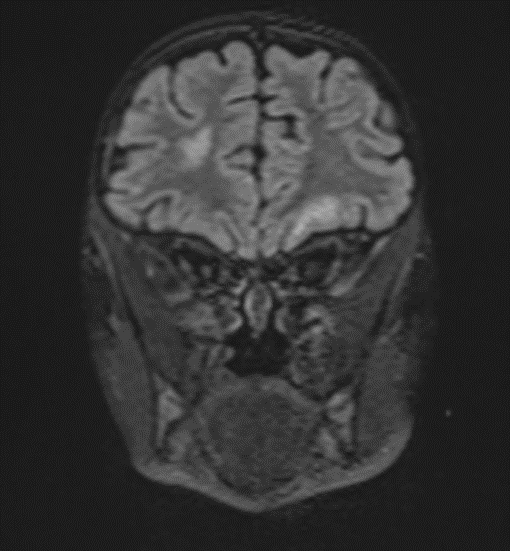
Picture1.
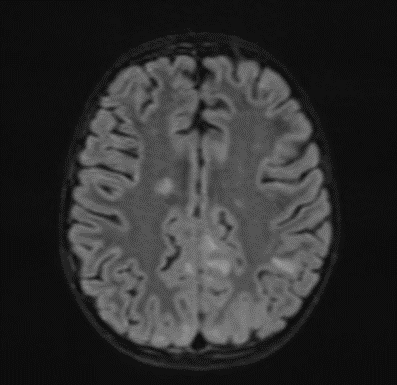
Subsequently, an MRI of the brain was performed, which demonstrated: Bilateral diffuse high signal changes in the cerebellum on T2 and FLAIR sequences, as well as areas of restricted diffusion, indicating focal zones of cytotoxic edema.
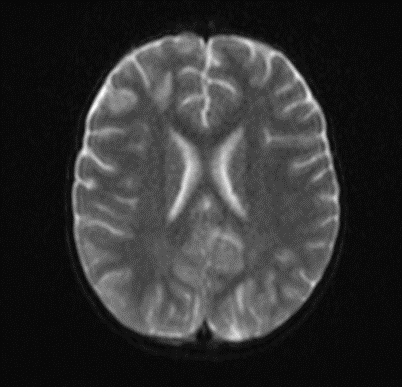
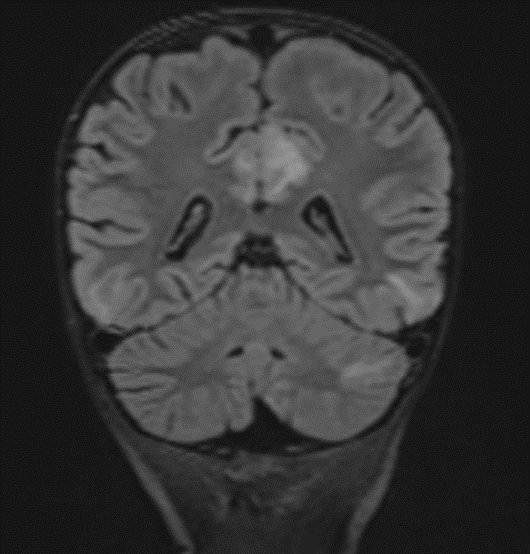
Picture 2.
The MRI of the brain revealed the following findings:
- Bilateral subcortical zones, predominantly in the occipital region, exhibiting confluent hyperintense signals on T2 and FLAIR sequences, accompanied by edema.
- A suspected area of cytotoxic edema in the superior parietal lobe.
- High signal intensities on T2 and FLAIR sequences observed in the centrum semiovale, right of the corpus callosum body, and bilaterally in the frontal gyrus, without diffusion restriction.
- In the supraventricular region, extending inferiorly from the projection of the postcentral gyrus into the subcortical white matter to the posterior part of the corpus callosum body (excluding it), and in the left superior temporal lobe, there was a cortico-subcortical lesion characterized by cytotoxic edema and restricted diffusion.
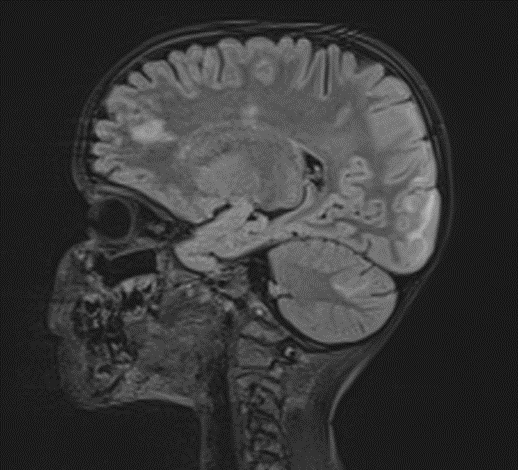
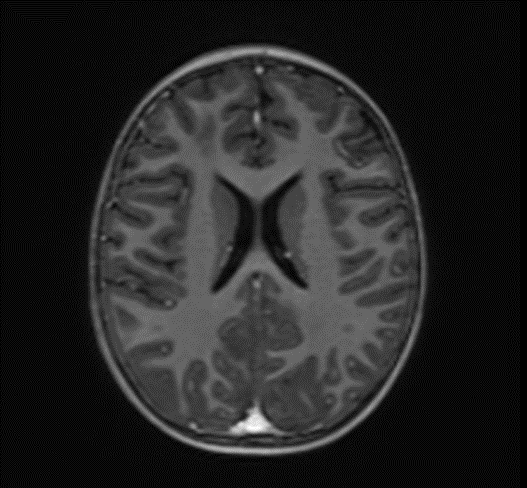
Picture 3.
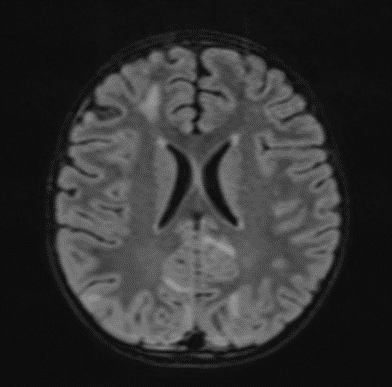
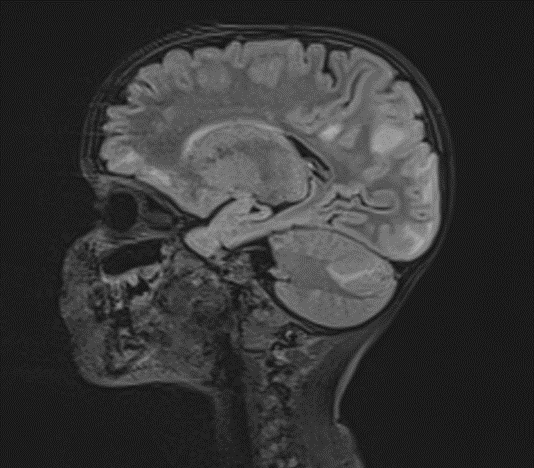
The changes observed are characteristic radiological markers appreciated on DWI, indicative of cytotoxic edema. The DWI changes are considered reliable early signs of acute toxic encephalopathy, specifically methotrexate-related leukoencephalopathy. Post-contrast MR images did not show any abnormal contrast enhancement.
Picture 4.
Discussion
Methotrexate (MTX) is a cornerstone chemotherapeutic agent used in the treatment of various malignancies, including Burkitt lymphoma. While effective, MTX is associated with several adverse effects, one of the most severe being methotrexate-related leukoencephalopathy (MTX-LE) (5). This case report highlights the radiological findings of MTX-LE in a pediatric patient diagnosed with Burkitt lymphoma, presenting with an abdominal manifestation and subsequently developing neurological symptoms.
Radiological Findings
MRI is instrumental in the early detection and evaluation of MTX-LE. In our case, MRI of the brain revealed the following key features:
Bilateral Occipital Dominant Subcortical Hyperintense Zones: These areas were evident on T2-weighted and FLAIR sequences, indicating diffuse cerebral involvement. The predilection for the occipital region was consistent with reported cases of MTX-LE, which often presents with symmetric white matter changes.
Edema and Cytotoxic Edema: The superior parietal lobes showed suspected zones of cytotoxic edema. This was further supported by the presence of diffusion-weighted imaging (DWI) changes, characterized by restricted diffusion, a hallmark of cytotoxic edema. These findings align with the pathophysiology of MTX-LE, where MTX-induced neuronal damage leads to cellular swelling and subsequent restricted diffusion.
Supraventricular and Frontal Gyrus Involvement: High signal intensities on T2 and FLAIR sequences were noted in the centrum semiovale, right of the corpus callosum body, and bilaterally in the frontal gyrus, without diffusion restriction. This suggests early involvement before the development of significant cytotoxic damage.
Cortico-Subcortical Lesion in the Left Superior Temporal Lobe: This region exhibited cytotoxic edema with restricted diffusion, underscoring the multifocal nature of MTX-LE. The involvement of both cortical and subcortical areas is a typical feature in severe cases of MTX-LE.
When encountering MRI findings suggestive of methotrexate-related leukoencephalopathy (MTX-LE), it’s essential to consider a broad range of differential diagnoses to ensure accurate diagnosis and appropriate management. Potential alternative explanation for similar radiological findings is Posterior Reversible Encephalopathy Syndrome (PRES). Although PRES typically manifests with posterior cerebral involvement, an atypical presentation may involve predominantly anterior regions. Awareness of these uncommon presentations is crucial for accurate diagnosis and appropriate management of PRES (6).
Clinical Implications
The early identification of MTX-LE is critical for timely intervention. The presence of characteristic DWI changes, even in the absence of clinical symptoms, should prompt consideration of MTX-LE (7). In this case, the patient’s neurological deterioration and convulsions underscored the need for immediate imaging and clinical intervention. The absence of abnormal contrast enhancement on post-contrast MR images suggests that MTX-LE can present without blood-brain barrier disruption, further complicating diagnosis without advanced imaging modalities.
Pathophysiology
MTX-LE is believed to result from direct toxic effects on oligodendrocytes and myelin sheaths, disruption of folate metabolism, and excitotoxicity mediated by homocysteine accumulation. The resultant demyelination and white matter necrosis lead to the observed radiological changes. The predilection for the periventricular white matter, as seen in this case, is characteristic of MTX-induced neurotoxicity.
Management and Prognosis
Upon diagnosis of MTX-LE, the immediate cessation of MTX is recommended, along with supportive care and consideration of alternative chemotherapeutic regimens (8). The prognosis of MTX-LE varies, with some patients experiencing partial or complete recovery, while others may suffer from persistent neurological deficits (9). Early detection, as highlighted in this case, is crucial for improving outcomes. The timing of a follow-up MRI is crucial for monitoring the progression or resolution of neurotoxicity and for guiding further treatment. Here are the general recommendations for follow-up MRI:
Immediate Follow-Up
Initial Follow-Up MRI: Typically recommended within 2 to 4 weeks after the initial diagnosis of MTX-LE. This helps to assess the progression or improvement of the lesions observed in the initial MRI and to evaluate the effectiveness of any interventions that have been initiated.
Mid-Term Follow-Up
Subsequent MRI: Depending on the findings of the initial follow-up MRI, a subsequent MRI might be performed every 1 to 3 months. The exact interval can be adjusted based on clinical symptoms, changes observed in previous imaging and the patient’s overall neurological status.
Long-Term Follow-Up
Long-Term Monitoring: After stabilization or improvement of the acute phase, MRI may be recommended every 6 to 12 months to monitor for any late-onset neurotoxicity or chronic changes, especially if the patient continues to receive chemotherapy or other neurotoxic treatments.
Clinical Triggers for MRI
Worsening Symptoms: Any new or worsening neurological symptoms, such as seizures, cognitive changes or motor deficits, should prompt an immediate MRI to rule out new or worsening leukoencephalopathy.
Reinitiation of MTX: If methotrexate therapy is reinitiated or modified, it may be prudent to perform an MRI before restarting and then at intervals during treatment to monitor for recurrence of neurotoxicity.
Conclusion
This case underscores the importance of vigilance for neurological symptoms in pediatric patients undergoing MTX therapy for Burkitt lymphoma. MRI, particularly DWI, plays a pivotal role in the early detection of MTX-LE. Recognizing the characteristic radiological markers can facilitate prompt diagnosis and intervention, potentially mitigating the severe neurological sequelae associated with this condition. Future studies are needed to further elucidate the pathophysiology of MTX-LE, and optimize management strategies for affected patients.
REFERENCES
- Walling J: From methotrexate to pemetrexed and beyond. review of the pharmacodynamic and clinical properties of antifolates. Invest New Drugs. 2006, 24:37-77. 10.1007/s10637-005-4541-1.
- Pui CH, Evans WE: Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006, 354:166-78. 10.1056/NEJMra052603.
- Ackland SP, Schilsky RL: High-dose methotrexate: a critical reappraisal. J Clin Oncol. 1987, 5:2017-31. 10.1200/JCO.1987.5.12.2017.
- Inaba H, Khan RB, Laningham FH, Crews KR, Pui CH, Daw NC: Clinical and radiological characteristics of methotrexate-induced acute encephalopathy in pediatric patients with cancer. Ann Oncol. 2008, 19:178-84. 10.1093/annonc/mdm466.
- Panicker V V, Radhakrishnan S E, Kuruttukulam G V, et al. (January 02, 2024) Methotrexate-Induced Leukoencephalopathy as a Clinical and Radiological Mimicker of Acute Ischemic Stroke Leading to Thrombolysis. Cureus 16(1): e51542. doi:10.7759/cureus.51542.
- Cancers (Basel). 2021 Apr; 13(8): 1939. Published online 2021 Apr 16. doi: 10.3390/cancers13081939Age- and Intravenous Methotrexate-Associated Leukoencephalopathy and Its Neurological Impact in Pediatric Patients with Lymphoblastic LeukemiaIlona Rijmenams,1,2,† Daan Moechars, Anne Uyttebroeck, Ahmed Radwan,4,5,6 Jeroen Blommaert,4 Sabine Deprez, Stefan Sunaert, Heidi Segers,2,3,4 Céline R. Gillebert,Jurgen Lemiere, and Charlotte Sleurs.
- K Wei, W Cao; (II) Administrative support: W Cao; (III) Provision of study materials or patients: K Wei, W Cao, B Yang, Y Liang, L Liu; (IV) Collection and assembly of data: K Wei, B Yang, L Liu; (V) Data analysis and interpretation: Y Liang, T Li, R Wang; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. ^ORCID: 0000-0002-1356-3705.
- Gaillard F, Sharma R, Baba Y, et al. Toxic leukoencephalopathy. Reference article, Radiopaedia.org (Accessed on 30 May 2024) https://doi.org/10.53347/rID-4437.
- Sharma R, Habana J, Thibodeau R, et al. Methotrexate-related leukoencephalopathy. Reference article, Radiopaedia.org (Accessed on 30 May 2024) https://doi.org/10.53347/rID-52847.