UDK: 615.015.2.03-053.8
TundzevaM.1, Velikj Stefanovska V.2, Stavrikj K.1
1Center for Family Medicine, Faculty of Medicine – Skopje, “Ss. Cyril and Methodius”
University – Skopje, Republic of North Macedonia
2Institute for Epidemiology and Biostatistics with Medical Informatics, Faculty of Medicine – Skopje, “Ss. Cyril and Methodius” University – Skopje, Republic of North Macedonia
Abstract:
Introduction: People with multimorbidity, who use several drugs at the same time, especially the elderly, are predisposed to a greater number of side effects.
Objective: The effectiveness of the STOPP/START instrument in primary care units in patients older than 65 years with multimorbidity and polypharmacy.
Material and method: A multicenter prospective randomized clinical trial conducted by 18 primary family physicians, during 12 months in 2022/ 2023. Patients older than 65 years with multimorbidity and polypharmacy were included. On 174 patients, an intervention with the STOPP/START instrument version 2 was initiated. The patients were screened for prescription at base line and after a period of 6 months after intervention.
Results: After using the STOPP/START instrument, 416 (29.19%) drugs were stopped in 162 patients (93.10%), with the highest proportion of stopping cardiac drugs (168 drugs in 104 patients), followed by psychiatric drugs – 96 (23.08%) in 86 (49.42%) patients. With the START intervention, 245 drugs were prescribed in 127 patients, with the highest proportion being cardiac drugs – 134 (54.69%) in 89 (51.15%) patients, followed by psychiatric drugs – 41 (16.73%) in 36 (20.69%) patients. With the STOPP/START instrument, 248 drugs and 12 OTC less than ZERO time were prescribed to the patients.
Conclusion: The implementation of appropriate interventions for polypharmacy affects can be reflected in reduction of negative effects from drug interactions, and thus reduction of unplanned outcomes
Key Words: elderly, multimorbidity, primary care, polypharmacy, STOPP/START intervention.
Introduction
Prescribing medicines is perhaps the most important intervention for people with multimorbidity (1). If multiple drugs are administered inappropriately, the term “Polypharmacy” is used (2). As a result, numerous adverse effects occur, which may lead to adverse events such as hospitalization or death (3). Deprescription is a systematic joint process of decisions and actions between the doctor and the patient, where the drugs are written off because they have no effect, that is, the harm is so great that it exceeds the benefit. STOPP, which is based on physiological systems, contains a list of 65 explicit rules for avoiding certain drugs. START is also systems-based and lists 22 common cases of potentially appropriate drugs and combinations in patients with specific medical problems (4, 5). In its second version published in 2015, the list includes revised criteria divided into groups depending on body systems in 14 green and red boxes with recommendations approved by 19 experts from 13 European countries (4).
These criteria cannot replace clinical judgment in individual cases but may serve to guide prescribing and description by physicians (5).
Material and Methods
The research is a multicenter prospective randomized study, which was conducted during 12 months in 2022/ 2023. The research included 174 respondents older than 65 years with multimorbidity (3 or more chronic diseases) and polypharmacy (5 or more drugs in the last 3 months) who were given an intervention with the STOPP/START instrument version 2.
The data obtained during the study were statistically analyzed using the SPSS software package, version 22.0 for Windows (SPSS, Chicago, IL, USA). The analysis of the qualitative series was done by determining the coefficient of relations, proportions and rates, and they were shown as absolute and relative numbers. The quantitative series were analyzed using measures of central tendency (mean, median, minimum values, maximum values), as well as measures of dispersion (standard deviation). Difference test was used to compare proportions. Spearman’s rank correlation coefficient was used to determine the relation between numerical variables and irregular frequency distribution. Univariate linear regression analysis was used to determine and quantify independent significant predictors of polypharmacy. A level of p<0.05 was considered to be statistically significant.
Results
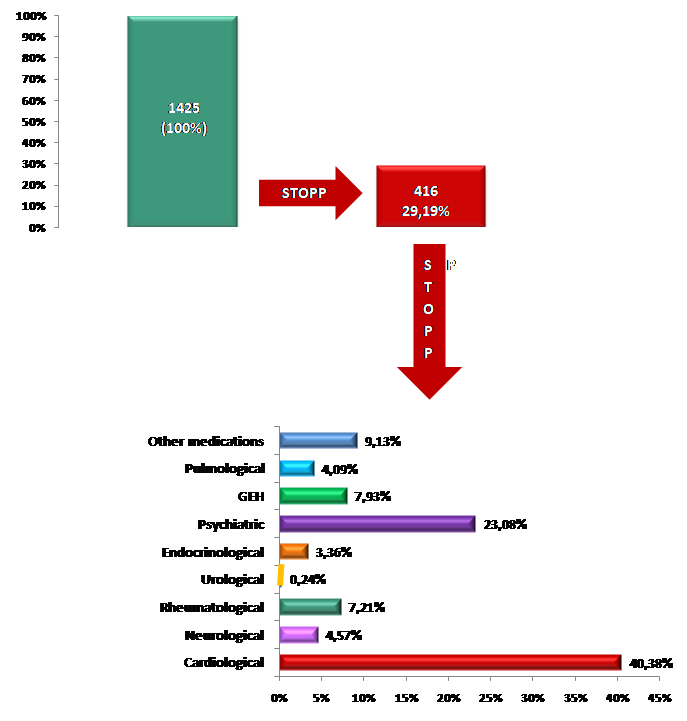
After the evaluation of therapy in patients with the STOPP/START instrument, 416 (29.19%) of 1,425 currently prescribed drugs were stopped. The change of drugs was made in 162 (93.10%) patients, during which a min/max of 0/7 drugs were stopped. The analysis of the total number of discontinued drugs/ supplements (N=416) indicated that (Table 1 and Graph 1):
Table 1. STOPP/START of drugs/supplements at ZERO time.
| Parameters
(ZERO time)
|
||||||
| STOPP | START | |||||
| Medications
N (%) |
Patients
N (%) |
Min/Max | Medications
N (%) |
Patients
N (%) |
Min/ Max | |
| Total | ||||||
| Medication/supplement | 416 (29.19%) | 162
(93.10%) |
0/7 | 245
(100%) |
127 (72.99%) | 0/5 |
| Types of medications | ||||||
| Cardiological | 168 (40.38%) | 104
(59.77%) |
0/3 | 134 (54.69%) | 89
(51.15%) |
0/4 |
| Neurological | 19
(4.57%) |
19
(10.92%) |
0/1 | 11
(4.49%) |
8
(4.60%) |
0/2 |
| Rheumatological | 30
(7.21%) |
28
(16.09%) |
0/2 | 11
(4.49%) |
8
(4.60%) |
0/2 |
| Urological | 1
(0.24%) |
1
(0.57%) |
0/1 | 1
(0.41%) |
1
(0.54%) |
0/1 |
| Endocrinological | 14
(3.36%) |
12
(7.70%) |
0/2 | 21
(8.57%) |
16
(9.19%) |
0/2 |
| Psychiatric | 96
(23.08%) |
86
(49.42%) |
0/3 | 41
(16.73%) |
36
(20.69%) |
0/2 |
| GEH | 33
(7.93%) |
32
(18.39%) |
0/2 | 16
(6.53%) |
15
(8.62%) |
0/2 |
| Pulmonological | 17
(4.09%) |
15
(8.62%) |
0/2 | 10
(4.08%) |
5
(2.87%) |
0/2 |
| Other medications | 38
(9.13%) |
31
(17.,82%) |
0/3 | 0
(0%) |
0
(0%) |
0/0 |
| GEH = Gastroenterohepatology
Mean = Average; SD = Standard deviation; Min/Max |
||||||
- Cardiac drugs had the largest proportion of stopped drugs/ supplements – 168 (40.38%) in 104 (59.77%) patients with a maximum of 3 drugs stopped per patient;
- The second largest proportion of stopped drugs/ supplements were psychiatric drugs – 96 (23.08%) in 86 (49.42%) patients with a maximum of 3 drugs stopped per patient;
- The proportion of other, GEH and rheumatological discontinued drugs/ supplements was 38 (9.13%) in 31 (17.82%) patients vs 33 (7.93%), in 32 (18.39%) patients vs 30 (7, 21%) in 28 (16.09%) patients respectively. The maximum number of discontinued drugs per patient was 3 for other drugs and 2 for GEH and rheumatological drugs each;
- Urological drugs had the smallest proportion of stopped drugs/ supplements – 1 (0.24%) in 1 (0.57%) patient with a maximum of 1 drug discontinued.
Graph 1. STOPP intervention of drugs/ supplements at ZERO time.
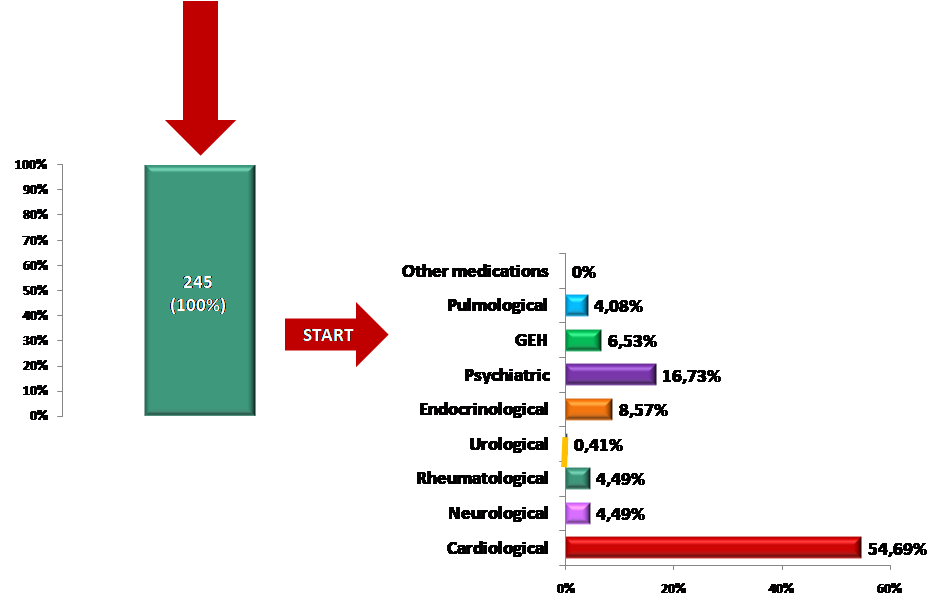
With the START instrument, 245 (100%) new drugs were prescribed in 127 (72.99%) patients with a min/max of 0/5 new drugs per patient. The analysis of the total number of started medicines/ supplements (N=245) indicated that (Graph 2):
- Cardiac drugs had the highest proportion of started drugs/ supplements – 134 (54.69%) in 89 (51.15%) patients with a maximum of 4 drugs started per patient;
- The second largest proportion of drugs/ supplements started were psychiatric drugs – 41 (16.73%) in 36 (20.69%) patients with a maximum of 2 drugs started per patient;
- Proportion of endocrinological and GEH drugs/ supplements started was 21 (8.57%) in 16 (9.19%) patients vs 16 (6.53%) in 15 (8.62%) patients respectively. The maximum number of drugs/supplements started was 2 for both endocrinological and GEH drugs;
- The assessment did not indicate the need to start other new drugs.
Graph 2. START intervention of drugs/ supplements at ZERO time.
State after STOPP/START intervention: After 6 months of the STOPP/START intervention, it was observed that 174 (100%) patients were prescribed a total of 1,177 drugs/ supplements or 248 drugs less than ZERO time. The average number of drugs prescribed after the STOPP/START intervention was 6.76±2.33 with a minimum of 3 and a maximum of 13 drugs. In 50% of the patients after the STOPP/START intervention, the number of prescribed medications was ≤6, and in 25%, <8 medications were prescribed. After the STOPP/START intervention, the average reduction in prescribed medications was 1.42±1.29 (Table 2).
Table 2. Analysis of the number of drugs/ supplements after STOPP/START intervention.
| Parameters | after STOPP/START | |||||
| Total
N (%) |
Consumers
N (%) |
Mean ± SD | Median (IQR) | Total difference1 | Consumers difference2 | |
| Medications | ||||||
| Medications | 1177 (100%) | 174
(100%) |
6,76±2,33 | 6 (5-8) | 248 | 248 |
| OTC | ||||||
| OTC | 136 (100%) | 83 (47,70%) | 0,78±1,05 | 0 (0-1) | 12 | 6 |
| Types of medications | ||||||
| Cardiological | 430 (36.53%) | 165 (94.82%) | 2,47±1,35 | 2 (2-3) | 69 | 2 |
| Neurological | 59 (5.01%) | 36
(20.68%) |
0,34±0,72 | 0 (0-0) | 22 | 6 |
| Rheumatological | 73 (6.20%) | 51
(29.31%) |
0,42±0,75 | 0 (0-1) | 14 | 17 |
| Urological | 57 (4.84%) | 38
(21.83%) |
0,33±0,66 | 0 (0-0) | 0 | -1 |
| Endocrinological | 208 (17.67%) | 121 (69,54%) | 1,19±1,04 | 1 (0-4) | 6 | 5 |
| Psychiatric | 65 (5.52%) | 48
(27.59%) |
0,37±0,67 | 0 (0-1) | 63 | 46 |
| GEH | 65 (5.52%) | 64
(36.78%) |
0,37±0,49 | 0 (0-1) | 16 | 14 |
| Pulmonological | 52 (4,42%) | 28
(16.09%) |
0,29±0,77 | 0 (0-0) | 13 | 6 |
| Other medications | 122 (10,36%) | 71
(40.80%) |
0,70±1,09 | 0 (0-1) | 25 | 5 |
| 1 Difference = Number of medications: Zero – STOPP/START; 2 Difference = Number of consumers: Zero – STOPP/START
OTC = Supplements; GEH = Gastroenterohepatology Mean = Average; SD = Standard deviation; Median; Min/Max; IQR=Percentiles |
||||||
After the STOPP/START intervention, the number of prescribed OTCs was 136 and it was reduced by 12 prescriptions, i.e. by 6 consumers. The average number of OTCs after STOPP/START was 0.78±1.05 with a min/max of 0/5 OTCs. In 50% of the patients after STOPP/START the number of prescribed OTCs was ≤1. After the STOPP/START intervention, the average reduction in prescribed OTCs was 0.07±0.42.
The largest proportion of reduced medications was in the CARDIOLOGY group (N=69) followed by PSYCHIATRIC (N=63), OTHER medications (N=25) and NEUROLOGICAL (N=22). The smallest proportion of reduced drugs was in the group of ENDOCRINOLOGY drugs (N=6), and there was no reduction in UROLOGY drugs (N=0) (Table 2).
The proportion of medicines’ users was highest among PSYCHIATRIC (N=46), RHEUMATOLOGY (N=17) and GEH (N=14). The smallest decrease in the number of consumers was for CARDIOLOGY drugs (N=2). There was an increase in the number of consumers by N=1 only for UROLOGY drugs.
Discussion
In our study, 416 (29.19%) out of 1,425 currently prescribed drugs were stopped after the assessment of therapy in patients with the STOPP/START instrument. The change of medications was made in 162 (93.10%) patients. Cardiac medications had the highest proportion of discontinued medications/ supplements, followed by psychiatric medications. The maximum number of discontinued drugs per patient was 3 for other drugs and 2 for GEH and rheumatological drugs each; urological drugs had the smallest proportion of stopped drugs/ supplements – with a maximum of 1 drug stopped.
With the START instrument, 245 (100%) new drugs were prescribed in 127 (72.99%) patients. The analysis of the total number of started drugs/ supplements (N=245) indicated that the highest proportion of started drugs/ supplements were cardiac drugs, followed by psychiatric drugs. The maximum number of drugs/ supplements started was 2 for both endocrinological and GEH drugs; the assessment did not indicate the need to start new other drugs.
After this intervention, 174 (100%) patients were prescribed a total of 1,177 drugs/ supplements or 248 drugs less than ZERO time. After the STOPP/START intervention, the number of prescribed OTCs was 136 and it was reduced by 12 prescriptions, i.e. by 6 consumers.
In the most recent study by Gareri et al. 2024 (6), the average number of drugs used in the sample was 9.4 drugs/ patient. The most common comorbidities were cardiovascular diseases (ischemic heart disease, hypertension, atrial fibrillation, heart failure). Potentially inappropriate drugs (PIM) were a total of 74 (36.1%) drugs. In ten patients, proton pump inhibitors (PPI) were stopped without a set indication, out of a total of (46.3%). In ten patients, over-the-counter drugs were prescribed (mostly supplements of the osteoarticular system, multivitamins), which were not necessary. The so-called “duplicate” or double drugs were 26 (12.7%) and were also discontinued. In another study (7), the STOPP/START identified a high prevalence of inappropriate drugs in elderly patients with advanced kidney disease (CKD), following which they were also discontinued. In the study by Ryan et al., the STOPP/START criteria identified a total of 346 PIMs prescribed for 284 (21.4%) patients. The most inadequate were drugs for the gastrointestinal system, in particular (PPI), followed by drugs whose primary effect is on the central nervous system, the musculoskeletal system and the cardiovascular system. Potentially inappropriate therapy associated with NSAIDs has been observed in patients with hypertension, osteoarthritis, and in patients with a history of peptic ulcer disease, gout, heart failure, CKD and dyspnea. A total of 333 (PPO) for 302 (22.7%) patients were identified with the START criteria. In a recent Japanese randomized controlled trial, the STOPP/START intervention was conducted in 106 participants (49.3%) in the intervention group, and 117 (51.5%) in the usual care group out of a total of 442 participants (average age 81.8 years) (9).
Conclusion
In our research, more than 90% of the respondents had medications stopped, mostly cardiac drugs, followed by psychiatric drugs. With the START intervention, all respondents from the sample were prescribed 250 drugs less than “Zero time”, primarily cardiac drugs, followed by psychiatric therapy. The STOPP/START instrument is a criterion for individual judgment for safe treatment, which would lead to rational prescription of drugs in people with multimorbidity.
Acknowledgements
WE are grateful to all participating professionals and respondents who contributed to the realization of this research. We would like to thank Biljana Tanevska Andonovska for communicating with the researchers, organizing meetings and collecting the materials.
Author Contributions
M.T. contributed to the conceptualization of the design and protocol and research, communication with other researchers, filling in data, formal analysis, project administration, supervision, writing and review of the original draft and keeping the data in original form. V.V.S. contributed to the statistical analysis, K.S. contributed to the conceptualization and protocol, supervision, validation, writing, reviewing and editing of the manuscript. The authors jointly agreed to write and publish the paper.
References
- Michalak M, Lewek P, Jankowska-Polańska B,et al . Polypharmacy Management in the Older Adults: A Scoping Review of Available Interventions. Front Pharmacol. 2021 Nov 26; 12:734045. doi: 10.3389/fphar.2021.734045. PMID: 34899294; PMCID: PMC8661120.
- Ermakov D, Fomina E, Kartashova O. Specific features of rational pharmacotherapy in elderly patients. Eur J Hosp Pharm. 2023 Nov;30(6):322-327. doi: 10.1136/ejhpharm-2021-002980. Epub 2021 Nov 18. PMID: 34795002; PMCID: PMC10647877.
- Cadogan, C.A., Ryan, C. & Hughes, C.M. Appropriate Polypharmacy and Medicine Safety: When Many is not Too Many. Drug Saf 39, 109–116 (2016). https://doi.org/10.1007/s40264-015-0378-5.
- O’Mahony D, O’Sullivan D, Byrne S,et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015 Mar;44(2):213-8. doi: 10.1093/ageing/afu145. Epub 2014 Oct 16. Erratum in: Age Ageing. 2018 May 1;47(3):489. doi: 10.1093/ageing/afx178. PMID: 25324330; PMCID: PMC4339726.
- Del Cura-González I, López-Rodríguez JA, Leiva-Fernández F, et al.MULTIPAP PLUS Group. Effectiveness of the MULTIPAP Plus intervention in youngest-old patients with multimorbidity and polypharmacy aimed at improving prescribing practices in primary care: study protocol of a cluster randomized trial. Trials. 2022 Jun 9;23(1):479. doi: 10.1186/s13063-022-06293-x. PMID: 35681224; PMCID: PMC9178530.
- Gareri P, Gallelli L, Gareri I,et al. Deprescribing in Older Poly-Treated Patients Affected with Dementia. Geriatrics (Basel). 2024 Feb 26;9(2):28. doi: 10.3390/geriatrics9020028. PMID: 38525745; PMCID: PMC10961769.
- Parker K, Bull-Engelstad I, Benth JŠ,et al. Effectiveness of using STOPP/START criteria to identify potentially inappropriate medication in people aged ≥ 65 years with chronic kidney disease: a randomized clinical trial. Eur J Clin Pharmacol. 2019 Nov;75(11):1503-1511. doi: 10.1007/s00228-019-02727-9. Epub 2019 Jul 29. PMID: 31359099.
- Ryan C, O’Mahony D, Kennedy J,et al. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol. 2009 Dec;68(6):936-47. doi: 10.1111/j.1365-2125.2009.03531.x. PMID: 20002089; PMCID: PMC2810806.
- Ie K, Hirose M, Sakai T,et al. Medication Optimization Protocol Efficacy for Geriatric Inpatients: A Randomized Clinical Trial. JAMA Netw Open. 2024 Jul 1;7(7):e2423544. doi: 10.1001/jamanetworkopen.2024.23544. PMID: 39078632; PMCID: PMC11289701.