UDK: 616-036.88-053.31:[618.3-06:616.379-008.64
Aleksandra Djordjevikj1, Dafina Karadjova1, Ivo Kaev1, Emilija Ivanov1, Nadica Mehmedovikj2
- University Clinic for Gynecology and Obstetrics, Skopje
- University Clinic of State Cardiac Surgery
Abstract
Introduction: Any degree of glucose intolerance with onset in pregnancy is referred to as gestational diabetes mellitus (GDM). It is present in 10% of all pregnancies, it has an increasing tendency and represents a risk factor for the mother, pregnancy and fetus. The aim of the paper is to determine the mutuality of the most common disorders in newborns from mothers with GDM, compared to the control group of newborns, from pregnancies without gestational diabetes in our maternity hospital.
Material and methods: Retrospective study, performed at the University Clinic for Gynecology and Obstetrics, in the period from 01.01. to 30.05.2024. The study included mothers with GDM, their newborns, as well as a control group of newborns and mothers without GDM. We evaluated maternal age, body weight, type of diabetes and comorbidities, maternal therapy, family history, way of delivery, Apgar scores, need for resuscitation, birth weight and maturity of the newborn, respiratory adaptation of the newborn, glycemia and needs for correction, hematocrit, Calcium, bilirubin, sucking reflex, jaundice.
Results: The study included 60 parturients, 30 with GDM and 30 parturients without diabetes. In the group of mothers with GDM, the mean age was 33.6 (20%), they had a positive family history of diabetes and hypertension, 26 (87%) were obese, 12 (40%) had high blood pressure, 25 (83%) gave birth by caesarean section. 33% of their newborns were premature, 33% hypertrophic, 13% had hypoglycemia, 10% in need of oxygen support during the adaptation period, 7% with hypocalcemia, 27% with prolonged hyperbilirubinemia. A weaker sucking reflex and weaker muscle tone were noted in half of the newborns of mothers with GDM.
Conclusion: With an increase in glucose intolerance and obesity in the young female population in the fertile period, GDM occurs as a frequent pathology after they become pregnant. Early screening in pregnancy plays a big role in reducing the consequences.
Key Words: gestational diabetes, newborn, neonatal morbidity.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease that is characterized by elevated glycemic values that over time lead to serious damage to the heart, blood vessels, eyes, kidneys and nerves. 422 million individuals in the world have diabetes, and every year 1.5 million die from the consequences of diabetes (WHO) (1).
According to WHO data for 2021, the prevalence of gestational diabetes mellitus (GDM) is highest in Southeast Asia 25.9%, followed by North America and the Caribbean 20.7%. Europe has a prevalence of 15% (1,2). More than half of the women with GDM will develop type 2 diabetes during 3-6 years after birth. It is present in 3-10% of pregnancies, and the consequences of GDM are affecting 18 million newborns per year. GDM is a typical example of how the mother’s disease has repercussions on the outcome of the pregnancy and the health of the fetus. Several factors are responsible for their teratogenic effect, among which the main role is played by variations in glycemia, insulin, blood pressure, obesity, mineral deficiency (zinc, calcium, iron), keto bodies, free radicals, but there are also factors that still are being investigated. More severe consequences after the pregnancy itself for the mother and the newborn are found in pre-gestational diabetes than in gestational diabetes, hence the importance of timely diagnosis and distinction of the type of diabetes, through screening methods in early pregnancy (3).
From a pediatric point of view, the most vulnerable period for the newborn from a mother with GDM (NGDM) is the period of organogenesis and the period of metabolic adaptation of the newborn. Physiological hypoglycemia is represented in 2-4% term, 5-10% preterm, but in 50% of newborns from mothers with GDM. Difficult temperature and respiratory adaptation are no less significant. Birth injuries, weaker muscle tone and sucking reflex, electrolyte deviations, jaundice, are part of the difficulties encountered by a large part of these newborns during the first days and even weeks after birth (3). These reasons give great importance to the early detection of diabetes (screening) and its regulation during pregnancy (4).
The aim of this study is to determine the characteristics of mothers with GDM and the representation of the most common disorders in their newborns, compared to a control group of newborns / mothers, in our maternity hospital.
Material and Methods
This is a retrospective study performed at the University Clinic for Gynecology and Obstetrics. The study included newborns in the period from 01.01. to 30.05.2024. Late preterm (340 – 366) and term newborns (370 – 416) from mothers with GDM and newborns from regular pregnancies (control group) were included. Early premature newborns, cardiorespiratory unstable newborns, ones with need for a higher degree of circulatory and respiratory support, newborns transferred to Intensive Care Unit were excluded from the study.
In mothers we analyzed age, body mass index (BMI), number of pregnancies, type of diabetes, hypertension disorders, type of therapy, family, personal and obstetric history (previous abortion, fetus mortus) and way of delivery.
In newborns we analyzed Apgar scores, need for neonatal resuscitation or oxygen support, gestational age of the newborn (in term >37 gestational week or preterm < 37 g.w.), weight, level of glycemia. For weight measurement we used WHO growth charts from 2009 and we classified newborns as eutrophic (AGA), hypotrophic (SGA) or hypertrophic (LGA). We measured weight without diapers on an electronic scale, while length was measured with a standard plastic meter. We also analyzed the need for non-invasive oxygen support due to signs of respiratory distress and O2 saturation <94%. If needed a tent with 0.015m2 volume, flow 1-5 l/min. with FiO2 <40%. was implemented.
Glycemia (mmol/L) was controlled by capillary blood with a glucometer (variation of 0.5-1 mmol/L below serum concentration). Possible inaccuracy of the glucometer at very low serum glycemic values in newborns was taken into account. Hypoglycemia was also confirmed by determining serum glycemic values. Skin-to-skin contact and breastfeeding were realized in the first 30-60 minutes from birth monitoring clinical signs of hypoglycemia (hypotonia, apnea or cyanosis, irritability). In the first 6 hours, the newborns had at least one meal, and in the first 24 hours at least 5 meals. Glycemia was controlled in a risk group of newborns (newborns from mothers with DM, late premature, hypotrophic, hypertrophic newborns) in the first 60 minutes after birth, 3-4 times daily during the first 24 hours, always serum glycemic values 30 minutes after meal. Newborns who were not in a risk group and without clinical signs of hypoglycemia, glycemia was controlled 2 and 4 hours after birth. Target glycemia in full-term newborns for the first 24 hours was over 2mmol/L, and in pre-term newborns was over 2.5mmol/L. Glycemia with values 2.2-2.4mmol/l was marked mild hypoglycemia, values 1.6-2.1mmol/l medium hypoglycemia and values <1.6mmol/l as severe hypoglycemia. If the results in all three controls in the first 24 hours were within reference limits, the further examination was stopped.
Recurrent hypoglycemia, with a serum glycemia value of 2-2.5mmol/l within 48 hours of birth, was corrected with nutrition (breastfeeding or milk formula), and with glycemia controls 3 times a day, 30 minutes after meal. In moderate hypoglycemia (glycemia of 1.5-2mmol/l) 10% Glucose 60-90ml/kg/day was given parenterally in addition to the diet. Severe hypoglycemia (glycemia below 1.5mmol/l) was corrected by tube feeding, with a bolus of 10% glucose 2.5ml/kg and 10% glucose 60-90ml/kg/day parenterally. In resistant hypoglycemia, 12% was given Glucose i.v. with previously taken blood for the investigation of insulin, cortisol and growth hormone.
Polycythemia (Hct >65%) was detected by determining the hematocrit (Hct) in a venous blood sample centrifuged at 3000rpm/ 4 min. When interpreting results, it was taken into account that hematocrit values increase in the first 2 hours after birth and spontaneously normalize after 6-24 hours. Hypocalcemia is a serum total calcium concentration of 2mmol/l in term infants or 1.75 mmol/l in preterm infants, or an ionized calcium concentration <0.75–1.10mmol/l. It was investigated in the first 48 hours of birth, in serum. Symptoms of hypocalcemia (hypotonia, poor feeding, tachycardia, tachypnea, convulsions) were closely monitored, and correction was carried out with oral administration of 10% calcium gluconate. Intravenous concentration of total, indirect and direct bilirubin (mmol/l) as a gold standard, Kramer’s rule, transcutaneous bilirubinometry, Bhutani nomogram, were tools in the daily control of jaundice in newborns of mothers with gestational diabetes and determining the need for therapy and type of therapy (non-invasive phototherapy / exanguinotransfusion).
Neurological symptoms in newborns were also noted.
Results
The study included 60 parturients, 30 with GDM and 30 in a control group – parturients without diabetes. The characteristics of the mothers are given in Table 1.
Table 1. Characteristics of examined mothers.
| Mothers with GDM | Control group of mothers | |||
| Average age (years) | 33 | 28 | ||
| Previous pregnancies | Abortion | 4 (13%) | 2 (7%) | |
| Fetus mortus | 2 (7%) | / | ||
| Family history | Diabetes and/ or hypertension | 6 (20%) | / | |
| Smoking | 4 (13%) | 6 (20%) | ||
| Body mass index (BMI, kg/m2) | normal <25kg/m2 | 4 (13%) | 22 (73%) | |
| overweight 25-29.9kg/m2 | 5 (17%) | 8 (27%) | ||
| obesity gr.1 30-34.9kg/m2 | 15 (50%) | / | ||
| obesity gr.2 35-39.9kg/m2 | 6 (20%) | / | ||
| Hypertension | 12 (40%) | / | ||
| Therapy | diet | 22 (73%) | / | |
| + antihyperglycemic | 6 (20 %) | / | ||
| +insulin | 2 (7%) | / | ||
| Way of delivery | Vaginal | In total | 5 (17%) | 20 (67%) |
| Induced vaginal | 5 (17%) | 3 (10%) | ||
| Cesarean section
|
In total | 25 (83%) | 10 (33%) | |
| Emergency cesarean section | 10 (33%) | 4 (13%) | ||
All neonates have good Apgar scores, in average Apgar score in first minute was 8, in 5th minute was 9, in 10th was 10. There was no need for neonatal resuscitation in any neonate.
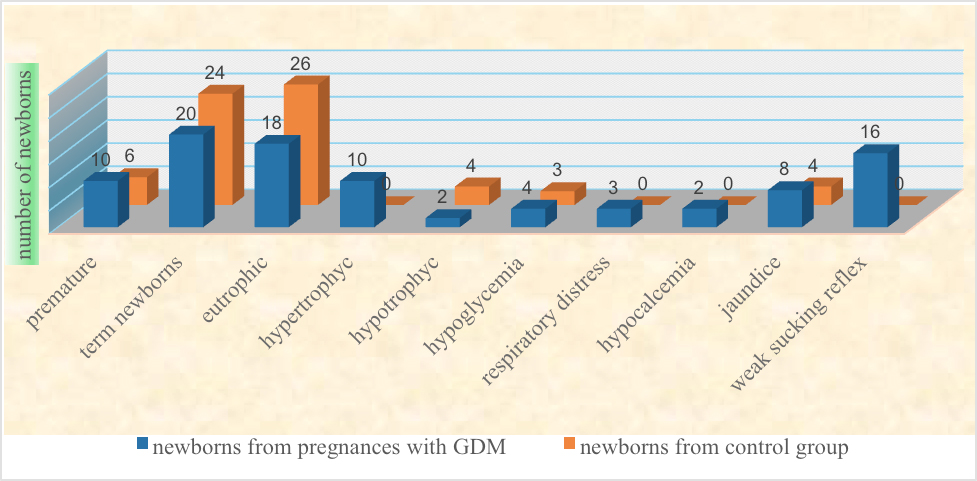
Regarding the gestational age of newborns, 10 neonates (33%) from mothers with GDM (NGDM) were late preterm, and 20 (67%) were term, while 6 neonates (20%) were late preterm and 24 were (80%) term newborns from mothers without GDM.
Regarding the weight, the largest number, 18 (60%) of neonates from mothers with GDM were eutrophic, in AGA class, 10 (33%) were LGA, hypertrophic, and 2 (7%) were SGA, hypotrophic. On the other hand, in neonates from mothers without GDM 26 (87%) were AGA, 4 (13%) were SGA, there were no LGA newborns in this group.
4 (13%) neonates from mothers with GDM had hypoglycemia, one of them was with refractory hypoglycemia, compared to 3 (10%) newborns with physiological hypoglycemia, from the control group.
3 (10%) newborns from the studied group developed respiratory distress, and need for non-invasive oxygen support, while in the control group of newborns, not a single newborn had respiratory distress.
Hematocrit in both studied groups did not deviate from reference values. Hypocalcemia was detected in 2 (7%) newborns from mothers with GDM, its presence was not detected in the control group. Hyperbilirubinemia in 8 (27%) NGDM, lasting 4 days with the need for intensive phototherapy, compared to 4 (13%) newborns from the control group. Neurological symptomatology in 16 (53%) NGDM was presented with a weak sucking reflex, which was not observed in a control group of newborns. No anomalies were detected in the two groups of newborns examined.
Table 2. Characteristics of examined newborns.
Discussion
Mothers
Our retrospective study showed a high association between overweight and obesity (87%) with GDM, which coincides with the results of many studies. Pirjani et al. in their prospective cohort study that followed 256 pregnant, obese and overweight patients, showed that 52.5% obese and 27.8% overweight developed GDM (5). In another observational study from Sunande et al., it was established that all pregnant women who in the first trimester of pregnancy had a BMI over 30kg/m2 had a significantly higher incidence of GDM compared to pregnant women with a normal weight (6). It is already established that the adipocytes function as endocrine glands with a wide effect on all organs, and through the release of leptin, TNF-alfa cytokines, FFA substrates play a major role in energy balance and glucose homeostasis, from which partly the connection between obesity and diabetes can be explained (7).
Hypertension as a complication in our group of subjects was detected in 40% of pregnancies with GDM. These results are higher than those in the comparative study of L. Sohonen, where the frequency of pregnancy-induced hypertension in mothers with GDM was 18.8% (8). HTA was well controlled with oral antihypertensives, which resulted in the absence of complications (in accordance with the recommendations of the Working Group for revision and adaptation of clinical guidelines and recommendations at the Ministry of Health of the Republic of Macedonia in 2018) (9).
In two-thirds of cases, glycemia was well controlled with a dietary diet in pregnant women with GDM, which resulted in good glycemic control and fetal growth. The percentage of mothers with GDM in whom for therapy oral antihyperglycemic drugs or insulin were given for glycemic control was small (27%). A Mediterranean diet is associated with improved glycemic control in women with GDM, and it was confirmed by the study of Fatemeh at al. (10).
According to our results, related to the way of delivery, operative deliveries prevailed in the GDM group, and one third of them were by emergency caesarean section. All patients in the same group had induced vaginal delivery. Compared to the results from the study of Sugiyama et al., the number of operative deliveries in our study was higher (11). The active management of labor and programmed early termination of birth in parturients with GDM as a risk group of pregnancies is in accordance with the recommendations of the American College of Obstetricians and Gynecologists (ACOG) (12). Witkop at al., in their systematic review, concluded that an active versus passive approach in choosing the term and method of delivery can reduce macrosomia and other complications in newborns and mothers with GDM (13).
Newborns
The results of our study showed that 33% of the examined group of newborns were premature, while 67% were in term. The detected higher incidence of preterm births refers to both operative births and spontaneous births, and it is a result of the risky pregnancy itself and the active management of it by obstetricians and programmed early termination of birth. Different studies showed different results, in most of them the values of preterm births are much lower. In Mohammed et al. study there were 92% full-term and 8% premature (14), in Giampiero et al. study 72% were term and 18% premature (15). Hedderson et al. in their cohort study of 46,230 pregnancies indicate the risk of spontaneous preterm births with increasing levels of glycemia in pregnancies, where all other factors that could affect the occurrence of premature birth were excluded (16).
In our study most of the newborns from mothers with GDM were eutrophic (60%). Despite promptly diagnosed and well-controlled glycemia, macrosomia was significantly increased in newborns from mothers with GDM compared to a control group, and the percentage of hypertrophic newborns was higher compared to published results from other studies. Swedish study from 2013 that compares 1,547 newborns from mothers with GDM and a control group of 83,000 newborns, reported an incidence of hypertrophies of 26% and 10.6% in the control group (17). A large cohort study (HAPO) confirms the strong association between the maternal level of glycemia and neonatal adiposity and suggest that it is a result of fetal hyperinsulinism, that is, the mediation of fetal insulin in neonatal adiposity as a growth factor (18). The reasons for this growth have been illuminated since the fifties, with Pedersen’s hypothesis, according to which there is a strong connection between the level of glycemia in the mother, fetal insulin production and neonatal macrosomia (19).
On the other hand, among our respondents we noted, in a smaller percentage, restriction in growth of newborns from mothers with gestational diabetes (6.7%). The finding coincides with the results of other studies (20). Intrauterine growth restriction in newborns of mothers with GDM is a result of a complex interaction of physiological, metabolic and molecular factors, and basically it is an interaction between hyperglycemia, microangiopathy, placental abnormalities and altered transport of nutrients (essential fatty acids).
Control of glycemia in term newborns and even more in preterm newborns represents a challenge for neonatologists. Daily fluctuation of glycemia (hyper/hypoglycemia) is also observed in this population and is difficult to monitor with traditional measurement of concentration in capillary blood, which delays the therapeutic intervention. Our results coincide with those published by other authors (21). In practice, there is no firm connection between glucose levels and neurological damage in newborns. Reversible and irreversible damages were detected even at higher glycemia levels than the target. An individual level of glycemia that can lead to neurological damage is difficult to determine. In our study, we were generally guided by the target values of the Royal Prince Alfred Hospital protocol (22).
10% of newborns in our study group needed non-invasive respiratory support, they were born at term, with a good Apgar score and thermostable. Our results roughly coincide with the results of a retrospective study by Kawakita et al. (23) who examined the risk of neonatal respiratory morbidity in 222,978 newborns of mothers without diabetes, with GDM and pregestational diabetes, and found an association between diabetes and increased risk for neonatal respiratory morbidity beyond that attributable to prematurity. Treatment of diabetes with insulin during pregnancy increases the risk of respiratory morbidity in newborns (due to the influence of surfactant and epithelial cells). The study of Becquet at al. on 18,095 term and late preterm infants from mothers without diabetes, with GDM and with pregestational diabetes on insulin therapy shows that the incidence of admission to the Intensive Care Unit due to respiratory distress was significantly higher in the group of newborns on insulin therapy (24).
Hyperbilirubinemia in newborns from mothers with GDM is one of the consequences in GDM pregnancies. The results of our study (newborns with jaundice and the need for intensive and prolonged phototherapy) are closer to the international rank of 20-25% and are higher than the published results of Mohammed at al. 4-years retrospective analysis (14).
A weak sucking reflex and hypotonus of a mild degree, clinically detected, in our subjects, was presented in half of newborns from mothers with GDM, for the entire time of stay in the maternity hospital. They are early symptoms of neuromuscular immaturity, as a result of disturbed metabolism of fats, carbohydrates, mineral deficiency. For the diagnosis of later consequences like autism spectrum disorders, motor disorders, memory function disorders, language development, intelligence, behavioral and psychological disorders, as well as epigenetic alterations, more extensive and far-reaching studies are needed. The incidence of these disorders tends to decrease by improving glycemic control in mothers (25).
Conclusion
GDM is a common pathology during pregnancy, and it tends to grow with the modern lifestyle.
By identifying women at risk of diabetes before becoming pregnant and advising them to change a lifestyle, nutrition and physical activity, its incidence can be significantly reduced.
Prenatal screening tests (OGTT), for timely detection of glucose intolerance, bring a great benefit to the mother, the outcome of the pregnancy and the newborn. Complications resulting from unrecognized, untreated and untreated metabolic syndrome have far-reaching consequences for the offspring.
Future research is needed with much bigger group of patients to get better recommendations.
References:
- Diabetes-World Health Organization (WHO) https://www.who.int>Health topic>Diabetes.
- International Diabetes Federation. IDF Diabetes Atlas 10th edition (IDF, 2021).
- Murali Mohan Voona. A study of complications in infants of Diabetic mother. Medical and Research Publication, 2023.
- A-M.H.Momsen, D.Hotoft, L.Ortenblad. Diabetes prevention interventions for woman after gestational diabetes mellitus: an overview of reviews. Journal List, Endocrinal Diabetes Metab. 2021;4(3).
- Pirjani R, Shirzad N, Qorbani M. Gestational diabetes mellitus its associated with obesity: a prospective cohort study. Eat Weight Disord. 2017;22(3):445-450.
- S.Bharatnur, P.B.Acharya. Association between maternal obesity and gestational diabetes melitus and their related outcomes. International Journal of Reproduction, Contraception, Obstetrics and Gynecology. 2023;12(10).
- B.B.Kahn, J.S.Flier, Obesity and Insulin resistance. The Journal of Clinical Investigation. 2000, 06(4):473-8.
- LSohonen, K.Teramo. Hypertension and pre-eclampsia in women with gestational glucose intolerance. Acta Obstet Gynecol Scand. 1993; 72(4):269-72.
- Министрство за здравство на Република Македонија, Медицина заснована на докази. Детекција на ризични состојби во бременост. Работна група за ревизија на клинички упатства и препораки htts://zdravstvo.gov.mk/wp-content/uploads/2019/03 .
- 10. M.Fatemeh, H.Fatemeh. Adherence to the Mediterranean diet and risk of gestational diabetes a prospective cohort study. BMC Pregnancy and Chilbirth. 2023:647.
- Sugiyuma Takashi et all. Pregnancy outcomes of gestational diabetes mellitus according to pre-gestational BMI in a retrospective multiinstitutional study in Japan. Endocrine Journal. 2014;61(4):373-380.
- The ObG Project, Update ACOG Guidance on Gestational Diabetes – When to deliver? 2023(01)02.
- C.T.Witkop, D.Neale, L.Wilson, E.Bass. Active compared with expectant delivery management in women with gestationl diabetes: a systematic review. Obstet Gynecol. 2009; 113:206-217.
- Mohammed H.Al-Qahtoni. Infant of diabetic mothers: 4 Years Analysis of Neonatal Care Unit in a Teaching Hospital, Saudi Arabia. Saudi J Med Med Sci. 2014; 2(3):151-6.
- C.Giampiero, G.Alessandra, T.Giulio. Materno-Fetal and Neonatal Complication of Diabetes in Pregnancy: A retrospective Study. J Clin.Med. 2020; 9(9):2707.
- M.M.Hedderson, A.Ferrara, D.A.Sacks, Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstetrics&Gynecology. 2003:102(4).
- H.Fadi, M.Person. Disproportionate body composition and neonatal outcome in offspring of mothers with and without gestational diabetes mellitus. Diabetes Care.2013; 36(11):3543-8.
- Metzger BE, Lowe LP, Dyer AR et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009; 58(2):453-9.
- J.Pedersen. Weight and length at Birth of Infants of Diabetic Mothers. Acta Endocrinologica. 1954; 16(4):330-342.
- MR Nateghi at al. Understanding the link Between Gestational Diabetes and intrauterine Growth restriction: A Comprehensive Narrative Review. Sarem Journal of Medicine Research. 2023;8(2):119-123.
- Z.Fasoulakis, A.Koutras, P.Antsaklis, M.Theodora et al. Interaction Growth Restriction Due to Gestational Diabetes: From Patophysiology to Diagnosis and Management. Medicina (Kaunas). 2023;59(6):1139.
- RPA Newborn Care Clinical Guidelines. 2024.
- T.Kawakita, K.Bowers, S.Hazarti et al. Increased Neonatal Respiratory Morbidity Associated With Gestational and Pregestational Diabetes: a retrospective study. Am J Perinatal. 2017;34(11):1160-1168.
- O.Becquet, F.El Khabbaz, C.Alberti et al. Insulin treatment of maternal diabetes mellitus and respiratory outcome in late-preterm and term singeltons. BMJ Open. 2015;5(6).
- Mishra V, Panigrahi N, Rao A, Huisman TA. Neurological Abnormalities in Infantsof Mothers with Diabetes Mellitus. Newborn. 2022;1(2):238–244.