UDK: 616.24-073.75
Petrovska T1,2, Cabukovska Radulovska J1,2, Sinokapovski S2, Kirkov Lj2
1PHI University Clinic of Surgery “St. Naum Ohridski” Skopje
2Faculty of Medical Sciences, University “Goce Delchev” Shtip
Abstract
Pulmonary arteriovenous malformation (PAVM) is rare, and it is often associated with Osler-Weber-Rendu syndrome. Clinical manifestations may be absent or present as chest pain, cough and hemoptysis. In our case, we are dealing with an asymptomatic patient. The diagnosis of this condition involves chest X-ray, CT scan of the chest, and in some institutions transthoracic contrast ultrasound. We present a case of 73-years-old patient with an incidental finding of pulmonary arteriovenous malformation.
Key Words: AV malformation, chest radiograph, CT lung.
Introduction
Pulmonary arteriovenous malformation (PAVM) is a rare condition characterized with bridging between an artery and a vein, which results with right-to-left shunt. This condition is most commonly a congenital anomaly of pulmonary arteries and veins. PAVM occurs in 20%-50% of patients with hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome).
Besides being a congenital anomaly, this condition can also occur as a consequence of surgical interventions, trauma, infections, hepatopulmonary syndrome, congenital heart diseases and metastases. The diameter of the feeding artery is an important parameter in the treatment of these patients. Transcatheter embolization with coils or plugs is possible in feeding arteries with a diameter of 3mm or more. If such finding is incidentally discovered, it requires follow-up with an appropriate protocol.
Clinically, some patients may present with hypoxemia, hemoptysis and nodules in the lungs. Rarely, this condition is asymptomatic.
Etiology and Epidemiology
This anomality is quite rare, with the Mayo Clinic reporting an incidence of 4.3 cases per year. In one study analyzing 21,000 MDCT scans capable of visualizing even very small nodules, the prevalence was 1 in 2,600 individuals. This condition is more common in females, with a ratio of 1.5-1.8 times higher compared to males. It is the most commonly accompanied by Osler-Weber-Rendu syndrome.
AV malformations grow slowly and rarely spontaneously disappear.
Clinical Findings: Symptoms caused by AV malformations are insidious due to their slow growth. Dyspnea, especially on exertion, may be present for an extended period. In severe cases, dyspnea at rest in an upright position may occur. Cyanosis may be present to a significant extent. Occasionally, hemoptysis may occur, although it is rare for it to be massive.
Sometimes patients may have headache, dizziness, syncope, tinnitus, diplopia, breast pain and cough. These symptoms are not clearly understood, but may be associated with hypoxemia, polycythemia or paradoxical embolization of AV malformations.
Differential Diagnosis
AV malformations need to be separated from other radiological findings, such as extravascular changes: granulomas, inflammations, hamartomas, metastases, as well as vascular changes: mediastinal fibrosis with venous collaterals, arteriovenous collaterals, hepatopulmonary syndrome, serpiginous blood vessels in pulmonary hypertension, tortuous venous vessels, venous varices. For example, granulomas appear as nodular shadows with small arterial blood vessels but lack venous vessels. They tend to calcify, and satellite granulomas may be present around them which is important in differential diagnosis with AV malformations.
Material and Methods
We present a case of 73-years-old patient who underwent MDCT of the lungs due to visualization of a lobulated shadow right paracardial on preoperative chest radiography. The patient did not exhibit signs of dyspnea, bleeding, or any occlusive changes of the blood vessels. Laboratory analyses were within normal limits.
On the chest radiograph, a shadow was visualized in the paracardial right area with lobulated contours. The surrounding parenchyma appeared normal, and the hila were unremarkable.
Image 1. Chest radiograph (PA and lateral view) showing a lobulated shadow in the right paracardial area.
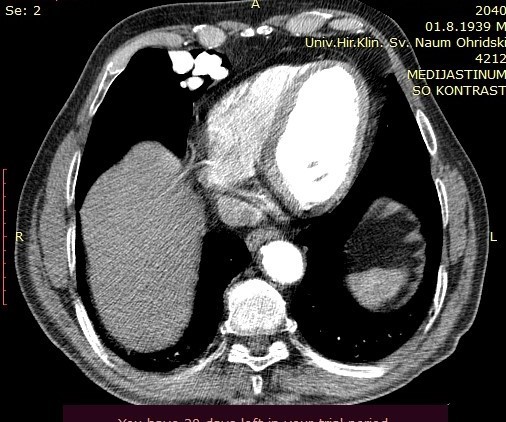
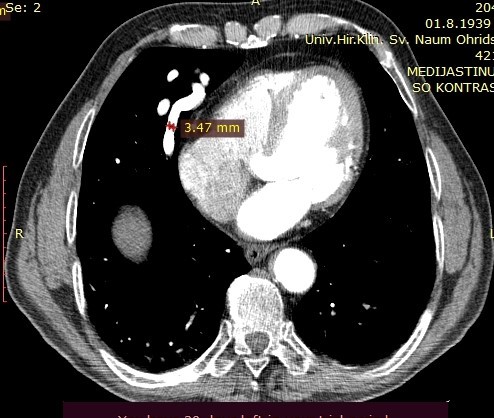
On the performed lung MDCT in the arterial phase, a fistula connecting arterial to venous vessels is clearly demarcated, with bridging of the normal capillary bed between them. The arterial blood vessel measures 3.47mm in diameter and may be a candidate for coil embolization.
Image 2. Lung MDCT exam, axial section, arterial phase, showing blood vessels in the right paracardial area.
Image 3. MDCT of the lungs, arterial phase, showing the pulmonary artery with a diameter of 3.47mm.
Image 4. MDCT of the lungs, contrast series in the coronal plane, showing a cluster of blood vessels.
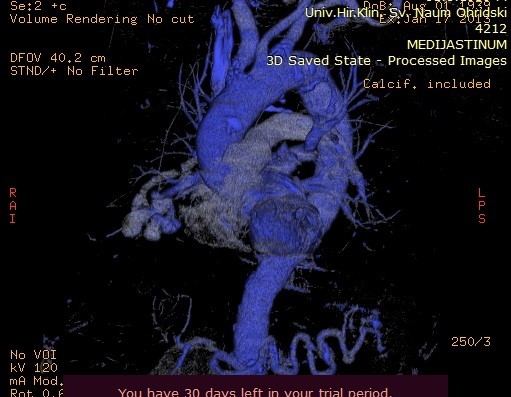
Image 5. Maximum Intensity Projection (MIP) reconstruction of the AV fistula at the level of arterial and venous blood vessels in the right lung.
Image 6. Maximum Intensity Projection (MIP) reconstruction showing AV shunting into the pulmonary arteries.
Conclusion
Pulmonary A-V malformation is rare condition as incidental finding. It the most often occurs with hereditary hemorrhagic telangiectasia. If there is any doubt about the extension of this condition, the correct diagnosis is essential for implementing an appropriate therapeutic procedure and avoiding complications such as the discharge of blood clots in distant organs.
References
1. Saboo SS, Chamarthy M, Bhalla S, et al. Pulmonary arteriovenous malformations: diagnosis. Cardiovasc DiagnTher 2018;8(3):325–337.
2. Cottin V, Chinet T, Lavolé A, et al. Pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: a series of 126 patients. Medicine (Baltimore) 2007;86(1):1–17.
3. Raptis A. D, Short R, Robb C, Marlow J, et all. CT Appearance of Pulmonary Arteriovenous Malformations and Mimics. RadioGraphics 2022 42:1, 56-68.
4.Danyalian A, Hernandez F. Pulmonary arteriovenous malformation. StatPearls [Internet]. 2020 Jan.
5.Majumdar S, McWilliams JP. Approach to pulmonary arteriovenous malformations: a comprehensive update. J Clin Med. 2020 Jun 19. 9(6).
6. Kjeldsen AD, Oxhoj H, Andersen PE, Elle B, Jacobsen JP, Vase P. Pulmonary arteriovenous malformations: screening procedures and pulmonary angiography in patients with hereditary hemorrhagic telangiectasia. Chest. 1999 Aug. 116(2):432-9.
7. Ragsdale JA. Hereditary hemorrhagic telangiectasia: from epistaxis to life-threatening GI bleeding. Gastroenterol Nurs. 2007 Jul-Aug. 30(4):293-9; quiz 300-1.
8. Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu- Osler-Weber syndrome). Am J Med Genet. 2000 Mar 6. 91(1):66-7.
9.Vase P, Holm M, Arendrup H. Pulmonary arteriovenous fistulas in hereditary hemorrhagic telangiectasia. Acta Med Scand. 1985. 218(1):105-9.
10. Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med. 1998 Aug. 158(2):643-61.
11. Halefoglu AM. Pulmonary arterio-venous fistula. In: Lang F, ed. Encyclopedia of Molecular Mechanisms of Disease. Berlin: Springer; 2009. 1759.
12. Dines DE, Arms RA, Bernatz PE, Gomes MR. Pulmonary arteriovenous fistulas. Mayo Clin Proc. 1974 Jul;49(7):460-5.
13. Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982-1997. Mayo Clin Proc. 1999 Jul;74(7):671-80.
14.White RI Jr, Lynch-Nyhan A, Terry P, et al. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology. 1988 Dec. 169(3):663-9.