Lozanovska B1, Sazdov D1, Derebanova Micunovic Lj2, Srceva Jovanovski M2
1Clinical hospital “Acibadem Sistina” Skopje, Republic of Macedonia, Department of Anesthesia and Intensive Care Medicine.
2University Clinic for Traumatology, Orthopedic Diseases, Anesthesia, Reanimation, Intensive Care and Emergency Center Skopje, Republic of Macedonia.
UDK: 616.831-001-053.2
https://www.doi.org/10.55302/MJA2481056l
Abstract
Traumatic brain injury is the leading cause of death and disability in children. Pediatric TBI is associated with several specific characteristics for the pediatric population that differ from adults. These differences are due to anatomical and physiological differences related to age, the mechanism of injury and difficulties in neurological evaluation. The specific pathological response to TBI results in distinctive accompanying neurological symptoms. Advances in technology and diagnostic methods have made diagnosis, treatment and prevention of complications easier. Better knowledge of the pathophysiology of traumatic brain injuries in childhood will provide a better basis for clinical management and treatment. This effort summarizes the results of recent relevant studies on this topic. Additionally, a clinical algorithm for the management and treatment of TBI in the pediatric population will be presented in accordance with the latest published data.
Key words: Algorithm, clinical management, pathophysiology, pediatric population, traumatic brain injury.
Definition
Traumatic brain injury (TBI) is defined as an injury to the head and brain caused by an external force that leads to impairment of brain function.
Epidemiology
According to the Centre for Disease Control and Prevention (CDC), nearly half a million children aged 0 to 14 admitted to emergency centers are due to TBI; 10% of the injuries are with a severe clinical picture (1). Mortality is higher in children under 4 years of age compared to those of 5 to 14 years of age. Head injuries are more common in male children and there is a 4 times higher risk of death compared to female children (2). The mechanism of injury depends on gender and age. According to the CDC, the most common cause of TBI is a fall from a height, then blunt force trauma, motorcycle accidents including bicycle accidents, fights, self-harm and sports injuries (3).
Anatomical and Physiological Characteristics of TBI in Pediatric Population
Children are more susceptible to TBI due to a larger head in relation to the body and weaker cranial bones, which provide less protection to intracranial structures. Additionally, they have a smaller subarachnoid space compared to adults, so a small increase in volume will lead to a large increase in intracranial pressure and incarceration. Less myelination in children leads to increased sensitivity to inflammatory mediators (4).
The cerebral metabolic rate of oxygen consumption is higher in children (5.2mL/100 gr/min of brain tissue) compared to adults, which makes them less tolerant to hypoxia (5). Autoregulation in neonates is maintained within a narrow mean arterial pressure (MAP) in range of 20–60mmHg. Beyond those limits, the relationship between Cerebral blood flow and systemic blood pressure is parallel. As a result, neonates are susceptible to brain ischemia and intraventricular hemorrhage (6).
Pathophysiology
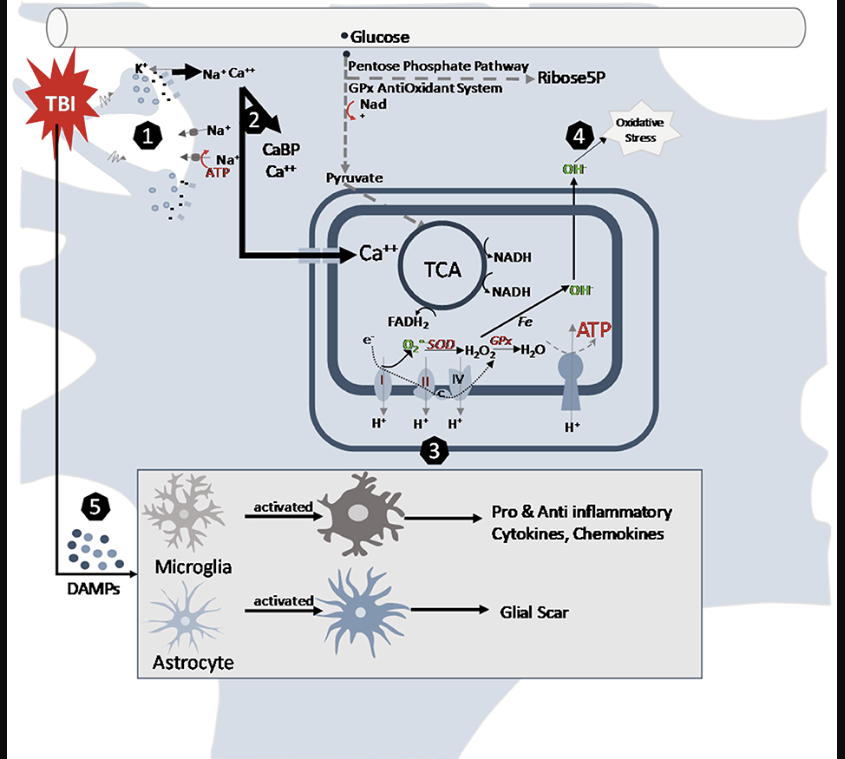
The consequences of neurotrauma depend not only on the primary, but also secondary injuries. Primary injuries are the result of the effect of direct force on intracranial structures and happen at the moment of impact. They lead to disruption of cell membranes and disturbance of the electrolyte balance. Elevation of intracellular calcium leads to activation of N-methyl-D-aspartate (NMDA) receptors, cell depolarization and accumulation of oxygen radicals that cause cell death. Secondary injuries, on the other hand, occur over minutes or days after the primary injury and are the result of chains of vascular, cellular and biochemical changes which lead to more pronounced inflammatory changes, edema, ischemia and necrosis (7).
Figure 1. Cellular pathways altered by traumatic brain injury in the juvenile brain (7).
Classification
Multiple classifications of head trauma in children can be found in the literature. This is the result of diverse causes and pathophysiologic mechanisms of different TBI that are not fully understood. Classification of TBI can be made according to severity and mechanism of injury. According to physical mechanism TBI can be classified as blunt and penetrating, and also as primary (when a direct hit or indirect-acceleration deceleration forces make the damage), and secondary which is the result of cellular and molecular damage, vasogenic and cytogenic edema (8). Another method of classification is through pathoanatomic characteristics. TBI can be divided into focal which is commonly the result of direct hit, and diffuse, which is due to acceleration deceleration forces. Focal injury can be contusion, hemorrhage, subarachnoid hemorrhage, subdural and epidural hematoma. Based on severity TBI can be classified according to the Glasgow Comma Scale score. GCS from 13 – 15 is classified as mild TBI, 9 – 12 as moderate TBI and 3-8 as a severe TBI (9). Classification of TBI can also be based on the duration of loss of consciousness. Children with 0 – 30 minutes of loss of consciousness can be classified as mild TBI, those between 30 minutes and 24 hours as moderate TBI, children with loss of consciousness more than 24 hours as having severe TBI.
Primary head injuries can be extra-axial, intra-axial and vascular. Examples of extra-axial head injuries are epidural and subdural hematoma, subarachnoid hemorrhage and intraventricular hemorrhage. Intra-axial injuries are contusion and cerebral hematoma and diffuse axonal injury. Vascular injuries can be pseudo aneurysm, dissection and different types of fistulas.
Secondary head injuries can be subdivided into acute and chronic. Acute injuries are brain edema, herniation, infarction and infection. Chronic injuries are for example hydrocephalus, cerebral tissue destruction and cerebrospinal fistula.
Diagnostics
The gold standard in the diagnosis of acute brain injury is Computed Tomography. But CT is also associated with radiation. Cancer incidence is significantly higher in children and adolescents who are exposed to CT (10). The physician needs to decide which child needs the CT scan of the brain. Several clinical decision-making tools (CHALICE, PECARN and CATCH) have been proposed and validated to help the decision-making process (11). Observation studies have shown that PECARN has the best sensitivity[i]. Another limitation of the CT scan consists in its low sensitivity in diagnosing Diffuse axonal injury (DAI). The patients with a Diffuse axonal injury, initially have normal CT scan. The findings of the initial CT scan do not correlate with the clinical picture where significant neurological symptoms and signs of elevated intracranial pressure are present. The subsequent CT scan is often positive and shows the secondary injury. On the other hand, MRI has greater sensitivity and specificity for DAI (12).
Clinical Picture
In the pediatric population multidisciplinary team approach is needed for effective clinical management. The management is through primary and secondary assessment. The primary assessment is conducted according to the A, B, C, D, E approach followed by a secondary assessment. During the secondary assessment, a history of comorbidities and diseases that are of interest to the patient is also taken. The Glasgow Coma Score is a commonly used tool for assessing consciousness. A modified version of the GCS for assessing consciousness in infants and children is shown in Table 1 (4).
Table 1. Modified Glasgow Coma Scale for infants and children.
In children with severe TBI, neurological deficit is also present initially. Symptoms are divided into early and late. Early symptoms (vomiting, headache, change of consciousness, neurological deficit, respiratory irregularities and convulsions) and late symptoms (Cushing reflex, paralysis of the III and IV cranial nerves, decerebrations and decortications and visual disturbances).
Factors that are associated with poor prognosis of pediatric patients with TBI are shown in Table 2 (11).
Table 2. Predictors of outcome in pediatric TBI are shown in the following table (11).
| Predictors |
| Age |
| High Injury Severity Score (ISS) |
| GCS ≤7 |
| Hypoxia (PaO2<60 mmHg) |
| Hypotension |
| Hyperventilation (PaCO2<35 mmHg) |
| Hyperglycemia (glucose >250 mg/dL) |
| Hyperthermia (>38°C) |
| Requirement for blood transfusion (≥20mL/kg) |
| Intracranial hypertension (ICP >20mmHg) |
| Cerebral perfusion pressure <40mmHg |
Airway Management Oxygenation Sedation and Ventilation Strategies
GCS≤8 is an indication for immediate endotracheal intubation and ventilation for airway protection, and management of elevated ICP. But the pediatric airway management represents a particular challenge. This is because of the anatomical and physiological differences compared to the adult airway. Younger children have a prominent occiput, so a pad placed under the thoracic spine provides neutral alignment of the spine. In this way, excessive flexion that may occur in the supine position is avoided. Additionally, because of uncertainty in the presence of concomitant craniospinal injury (CSI), cervical immobilization is necessary until it is excluded by clinical and radiological investigations to avoid compression of the spinal cord and worsening of neurological injury. It is best to use a combination of several techniques to stabilize the cervical spine, such as a spine board, forehead strap, sandbags. For children over 6 months of age, a rigid cervical collar can be used. The presence of blood and regurgitated masses in the mouth, injuries of the larynx and pharynx and intracranial hypertension, can also cause complications in airway management. Inadequate assessment of respiratory status can lead to inadequate resuscitation, deterioration and subsequent hypoxia. All patients with TBI who require endotracheal intubation are assumed to have a full stomach and thus undergo rapid sequence induction. Extreme care should be taken during laryngoscopy and intubation in order to avoid neck displacement. A video laryngoscope can also be used to prevent hemodynamic instability and ICP elevation. Nasotracheal intubation should be avoided if there are basilar skull fractures. Hypoxemia (PaO2<60mmHg) has deleterious effects in children with TBI because of the linearly increase of cerebral blood flow, cerebral blood volume and intracranial pressure. It was previously shown that it is an independent predictor of mortality. Therefore, all available measures should be taken to prevent it and correct it. Although in adults positive end-expiratory pressure (PEEP) has been shown to increase intracranial pressure (ICP), in children the optimal PEEP is not established. Hyperventilation can be used to decrease ICP and delay possible herniation, but only as a temporary measure until definitive management because it leads to cerebral vasoconstriction, reduced cerebral blood flow CBF and cerebral hypoxia (13). If clinicians decide to hyperventilate the pediatric patient, advanced neuromonitoring should be used to monitor for possible cerebral ischemia. The patients with TBI are at risk for lung injury, which leads to the use of lung protective ventilation.[ii] Studies do not show reduction in mortality or length of mechanical ventilation in these patients. The cornerstone is still to maintain normocapnia and prevent hypoxia (14).
When managing the airway of child with the appropriate sedative agent and its dose should be carefully considered in order to facilitate airway intervention, while maintaining both mean arterial pressure (MAP) and cerebral perfusion pressure (CPP) (15). Benzodiazepines, propofol etomidate, ketamine, have all been used and studied in patients with TBI. Their advantages and disadvantages are well documented. No single sedative agent has been shown to be better for adult patients with severe TBI, when compared to functional recovery, management of increased ICP or mortality (16). Therefore, in children with known adrenal insufficiency, etomidate may the preferred agent. For hemodynamically unstable patients, ketamine can be used, and in patients where benzodiazepines and propofol are used, care must be taken to prevent and correct hypotension.
Circulatory Support Coagulation and Transfusion
TBI in children is often accompanied by hypotension and anemia, which as hypoxia, is also associated with poor outcome because of reduced oxygen delivery. There are several mechanisms for hypotension such as acute traumatic coagulopathy (ATC), internal and external hemorrhage and neurogenic hypotension. Acute traumatic coagulopathy is the direct effect of trauma and can be exaggerated with hemodilution with fluid resuscitation, hypoperfusion, hypothermia, acidosis and hypocalcemia. Risk factors for ATC in children include GCS ≤8, increasing age, higher disease severity and brain contusion/laceration (17). If ICP monitoring is not used and there is a suspicion of its elevation, it is recommended to maintain slightly elevated blood pressure in order to sustain adequate cerebral perfusion pressure (CPP) (18). For that purpose, Isotonic crystalloid solutions are used the most often for resuscitation. Hypertonic saline has the advantage of increasing arterial pressure with a small volume in patients where fluid overload is a concern. A transfusion of red blood cells is recommended if hemoglobin level is below 8.0g/dl in children with TBI (19). In cases where adequate mean arterial pressure cannot be achieved with fluids, only concomitant intravenous vasopressors like noradrenaline and phenylephrine should be initiated (20).
Glycemic Control, Nutrition and Steroids
Hyperglycemia is another predictor for poor outcome in pediatric TBI. Recent studies show that the presence of hyperglycemia in the first 24 hours after admission is associated with increased length of stay in intensive care, increased duration of mechanical ventilation and increased mortality (21). Causes of hyperglycemia after TBI include increased gluconeogenesis and glycogenolysis, activation of the autonomic nervous system, activation of inflammatory cytokine pathways, pituitary and adrenal disfunction. Therefore, Dextrose as a solution for resuscitation should be avoided, except in established hypoglycemia. Hyperglycemia should be treated with caution because of the potential for hypoglycemia and neurologic sequelae if not recognized and treated. Given the possible negative effects of both hypo and hyperglycemia, Intermittent monitoring of blood glucose intraoperatively is recommended (21). Caloric expenditure in these patients may be double the expected resting energy expenditure. Increased caloric needs are result of increased temperature, muscle tone, GCS and measurement time associated with the injury. There is a significantly higher mortality rate as a result of undernourishment in the two-weeks’ post-injury period compared to receiving full nutrition for 7 days. The patients with adequate nutritional support have been shown to have less infections and complications. There for nutritional support should start slowly during the first 48 hours with the aim to achieve full nutritional support by day seven from trauma (22). Use of steroids is accompanied by complications like hyperglycemia, infection, bleeding from the gastrointestinal tract. A large multicenter study showed no benefit of their use (23).
Temperature Control
Recent studies have shown no benefit from moderate hypothermia (32–33°C) versus normothermia in children with severe TBI (24,25). According to the recommendations, moderate hypothermia begins within a time frame of 8 hours after severe TBI lasting 48 hours, and can serve as a neuroprotective measure, as well as for refractory intracranial hypertension. A warming rate greater than 0.5°C should be avoided because it is complicated with cardiovascular instability, increased ICP, coagulopathy and sepsis. Hyperthermia, on the other hand, worsens the prognosis after TBI by increased metabolic demand, lipid peroxidation, inflammation and seizure reduction (26).
Intracranial Pressure and Neuromonitoring
Normal intracranial pressure in children and newborns ranges from 3-7mmHg and 1.5-6 respectively (27). Elevation of ICP can be the result of traumatic intracranial process, vascular swelling and/ or cerebral edema. The recommendations state that immediate intervention is needed at intracranial pressure (ICP) >20mmHg. Values of cerebral perfusion pressure above 43, 54 and 58mmHg in children aged 2-6, 7-10, 11-16, are associated with a better outcome (28). Intracranial pressure monitoring may be considered for children with abnormal KTM and initial GCS <8. Despite the recommendations, ICP monitoring is used only in 7.7 to 55% of children with severe craniocerebral injury with GCS 3. In those cases, an improvement in the outcome was also observed, but with a significantly longer stay in the hospital, more days on a respirator. Common measures that are used to reduce ICP are placing the head in a position of 30°, sedation and analgesia, intubation and controlled mechanical ventilation, CSF-drainage, diuretics, osmotherapy (mannitol, HS), controlled hyperventilation. In refractory cases, barbiturate coma and moderate hypothermia, surgical decompression may be helpful (29).
Prophylaxis of Convulsions
Post-traumatic convulsions in childhood occur in 19% of the cases. Most often in the first 24 hours after the injury. Their consequences are multiple such as deepening and prolongation of hypoxia, increased release of excitotoxic neurotransmitters, increase in oxygen consumption rate and ICP and fluctuations in systemic blood pressure. Age under 2 years, nonaccidental trauma, GCS<8, skull fracture, subdural hematoma, are associated with occurrence of seizures in the first 7 days after injury. The use of phenytoin is recommended for the prevention of early post-traumatic convulsions (30).
Conclusion
Neurotrauma continues to be a leading cause of mortality in pediatric patients worldwide. More and more children are at risk of traffic accidents as passengers, pedestrians and cyclists. Children have a unique pattern of injury and response to it. It is necessary to move towards a more sophisticated algorithm in the care of such patients based on the unique pathophysiology of each individual.
References:
- Peterson AB, Zhou H, Thomas KE, Daugherty J. Traumatic brain injury-related hospitalizations and deaths by age group, sex, and mechanism of injury: united states 2016/2017.
- Greene NH, Kernic MA, Vavilala MS, Rivara FP. Variation in pediatric traumatic brain injury outcomes in the United States. Archives of physical medicine and rehabilitation. 2014 Jun 1;95(6):1148-55.
- Greene NH, Kernic MA, Vavilala MS, Rivara FP. Variation in pediatric traumatic brain injury outcomes in the United States. Archives of physical medicine and rehabilitation. 2014 Jun 1;95(6):1148-55.
- Araki T, Yokota H, Morita A. Pediatric traumatic brain injury: characteristic features, diagnosis, and management. Neurologia medico-chirurgica. 2017; 57(2):82-93.
- Ragan DK, McKinstry R, Benzinger T, Leonard J, Pineda JA. Depression of whole-brain oxygen extraction fraction is associated with poor outcome in pediatric traumatic brain injury. Pediatric research. 2012 Feb;71(2):199-204.
- Pryds O. Control of cerebral circulation in the high‐risk neonate. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1991 Sep;30(3):321-9.
- Lui A, Kumar KK, Grant GA. Management of Severe Traumatic Brain Injury in Pediatric Patients. Frontiers in toxicology. 2022 Jun 24; 4:910972
- Greve MW, Zink BJ. Pathophysiology of traumatic brain [iii] Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine: A Journal of Translational and Personalized Medicine. 2009 Apr; 76(2):97-104.
- Capizzi A., Woo J., Verduzco-Gutierrez M. (2020). Traumatic Brain Injury. Med. Clin. N. Am. 104 (2), 213–238. 10.1016/j.mcna.2019.11.001.
- Hawryluk G. W. J., Manley G. T. (2015). Classification of Traumatic Brain Injury. Handb. Clin. Neurology, Traumatic Brain Inj. Part I., 15–21. Elsevier. 10.1016/b978-0-444-52892-6.00002-7.
- Kulesza B, Nogalski A, Kulesza T, Prystupa A. Prognostic factors in traumatic brain injury and their association with outcome. Journal of Pre-Clinical and Clinical Research. 2015;9(2).
- Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013; 346:f2360.
- Dunning J, Daly JP, Lomas JP, et al. Derivation of the children’s head injury algorithm for the prediction of important clinical events decision rule for head injury in children. Arch Dis Child 2006; 91:885-91.
- Babl FE, Borland ML, Phillips N, et al. Accuracy of PECARN, CATCH, and CHALICE head injury decision rules in children: a prospective cohort study. Lancet 2017; 389:2393-402.
- Dayan PS, Ballard DW, Tham E, et al. Use of Traumatic Brain Injury Prediction Rules With Clinical Decision Support. Pediatrics 2017; 139:e20162709.
- Smitherman E, Hernandez A, Stavinoha PLet al. Predicting outcome after pediatric traumatic brain injury by early magnetic resonance imaging lesion location and volume. J Neurotrauma. 2016; 33:35-48.
- Kochanek PM, Tasker RC, Carney N, Totten AM, Adelson PD, Selden NR, Davis-O’Reilly C, Hart EL, Bell MJ, Bratton SL, Grant GA, Kissoon N, Reuter-Rice KE, Vavilala MS, Wainwright MS. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines. Pediatr Crit Care Med. 2019 Mar;20(3S Suppl 1):S1-S82. doi: 10.1097/PCC.0000000000001735. Erratum in: Pediatr Crit Care Med. 2019 Apr;20(4):404. PMID: 30829890.
- Luo XY, Hu YH, Cao XY, et al. Lung-protective Ventilation in Patients with Brain Injury: A Multicenter Cross-sectional Study and Questionnaire Survey in China. Chin Med J (Engl) 2016; 129:1643-51.
- Agrawal S, Branco RG. Neuroprotective measures in children with traumatic brain injury. World J Crit Care Med 2016; 5:36-46.
- Flower O, Hellings S. Sedation in traumatic brain injury. Emerg Med Int 2012; 2012:637171.
- Roberts DJ, Hall RI, Kramer AH, et al. Sedation for critically ill adults with severe traumatic brain injury: a systematic review of randomized controlled trials. Crit Care Med 2011; 39:2743-51.
- Epstein DS, Mitra B, O’Reilly G, RosenfeldJV, Cameron PA. Acute traumatic coagulopathy in the setting of isolated traumatic brain injury: A systematic review and meta-analysis. Injury. 2014; 45:819-82.
- Williams M, Lee JK. Intraoperative blood pressure and cerebral perfusion: Strategies to clarify hemodynamic goals. Paediatr Anaesth. 2014; 24:657-667.
- Acker SN, Partrick DA, Ross JT, NadlonekNA, Bronsert M, Bensard DD. Blood com-ponent transfusion increases the risk ofdeath in children with traumatic brain injury. J Trauma Acute Care Surg. 2014;76:1082-1087.
- Sookplung P, Siriussawakul A, Malakouti Aet al. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocrit Care. 2011; 15:46-54.
- Fu YQ, Chong SL, Lee JH, et al. The impact of early hyperglycaemia on children with traumatic brain injury. Brain Inj 2017; 31:396-400.
- Lui A, Kumar KK, Grant GA. Management of Severe Traumatic Brain Injury in Pediatric Patients. Frontiers in toxicology. 2022 Jun 24; 4:910972.
- Elliott E, Shoykhet M, Bell MJ, Wai K. Nutritional support for pediatric severe traumatic brain injury. Frontiers in Pediatrics. 2022 May 17; 10:904654.
- Edwards P, Arango M, Balica L et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005; 365:1957-1959.
- Crompton EM, Lubomirova I, Cotlarciuc I, et al. Meta-Analysis of Therapeutic Hypothermia for Traumatic Brain Injury in Adult and Pediatric Patients. Crit Care Med 2017; 45:575-83.
- Beca J, McSharry B, Erickson S et al. Hypothermia for traumatic brain injury in children—A phase II randomized controlled trial. Crit Care Med. 2015; 43:1458-1466.
- Lewis SR, Evans DJ, Butler AR, et al. Hypothermia for traumatic brain injury. Cochrane Database Syst Rev 2017; 9:CD001048.
- Dunn LT. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002; 73(Suppl 1):i23-i27.
- Chambers IR, Stobbart L, Jones PA et al.Age-related differences in intracranial pressure and cerebral perfusion pressure in the first 6 hours of monitoring after children’s head injury: Association with outcome.ChildsNerv Syst. 2005; 21:195-199.
- Carney, N., Totten, A. M., O’Reilly, C., Ullman, J. S., Hawryluk, G. W. J., Bell, M. J., et al. (2017). Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 80 (1), 6–15. doi:10.1227/neu.0000000000001432.
Chung MG, O’Brien NF. Prevalence of early post traumatic seizures in children with moderate to severe traumatic brain injury despite levetiracetam prophylaxis. PediatrCrit Care Med. 2016; 17:150-156.