Donev L1,2, Litajkovska S2,3, Gavrilovska-Brzanov A1,2, Aleksovski Z3, Trojacanec J2, Jovanovski-Srceva Marija1,2
1University Clinic for Anesthesia, Reanimation and Intensive Care Medicine, Skopje, Republic of North Macedonia
2University “Ss. Cyril and Methodius”, Skopje, Republic of North Macedonia
3University Clinic for Pediatric Surgery, Skopje, Republic of North Macedonia
UDK: 616-085.277-089.819.1-06-053.2
https://www.doi.org/10.55302/MJA2481026d
Abstract
Introduction: Modern chemotherapy protocols in pediatric oncology necessitate the deployment of central venous catheters. The duration of catheter usage ranges from several months to a year. Typically, long-term central venous catheters (LCVC), including external tunneling (ETLCVC) and totally implanted (TILCVC) variations, are employed for this purpose.
Aim: This study aims to assess the superiority of ETLCVC over TILCVC and to evaluate the occurrence of delayed complications, specifically focusing on catheter occlusion, dislocation of the catheter and catheter – related thrombosis, following the placement of central venous catheters.
Material and Methods: This prospective interventional clinical study encompassed 120 pediatric patients, aged 2-14 years, diagnosed with leukemia, lymphomas and solid tumors. The participants were stratified into two groups (n=60). Group 1 received ETLCVC, whereas Group 2 underwent TILCVC placement. An informative interview was conducted with the eligible patients’ parents, and written consent for study participation was obtained.
Results: In the group of patients with an implanted ETLCVC, four patients had a dislocation of the catheter, three patients had a catheter occlusion, two patients had a catheter – related thrombosis. In the group of patients in whom a TILCVC was implanted, one patient had an dislocation of the catheter, one patient had catheter occlusion, and there wasn’t any patient with catheter – related thrombosis.
Conclusion: Incidence of delayed complications (catheter occlusion, dislocation of the catheter, and catheter – related thrombosis) in our study were more frequent in patients in whom ETLCVC was applied, but this difference did not have statistical significance.
Key Words: Delayed complications from long-term central venous catheters, long-term implanted central venous catheters, pediatric oncology patients.
Introduction
Pediatric patients diagnosed with malignant diseases represent a rare occurrence, yet approximately 80 cases are reported annually in our country, necessitating the placement of a long-term central venous catheter (1). These diseases rank as the second most common cause of death in patients aged 12 months and older (2). The psychological impact of these illnesses can contribute to future complications, underscoring the importance of understanding how young patients cope with their conditions and the duration of their treatment.
In the field of pediatric hemato-oncology, various types of fully implanted long-term central venous catheters, such as external tunneling and totally implanted catheters, are employed (3). The selection of an appropriate catheter for pediatric oncology patients is influenced by their specific needs, taking into account factors like planned treatment duration, anticipated catheter usage and institutional capacity (4). It is recognized that cytostatic drugs exert a vascular irritating effect, placing a progressive strain on the venous system. Consequently, frequent peripheral venipunctures can induce behavioral and physiological reactions linked to pain or anxiety (4).
Due to the inadequate peripheral venous vascular system in children, it becomes necessary to insert a long-term central venous catheter for secure central venous access during cytostatic treatment (5). Given that the duration of treatment can extend from several weeks to several years, especially in cases like leukemia where the process lasts an average of two years, long-term central venous catheters play a crucial role in facilitating the daily administration of chemotherapy. These catheters serve as essential tools for safely infusing chemotherapeutic agents, supportive medications, blood products, hydration, and total parenteral nutrition (6). By avoiding frequent painful punctures of peripheral veins, long-term central venous catheters are believed to alleviate daily stress and enhance the quality of life for pediatric patients (7,8).
Pediatric oncology patients with long-term central venous catheters, require specialized treatment. The placement of central catheters can be accomplished through various techniques, including “blind” percutaneous venipuncture guided by anatomical landmarks, percutaneous puncture with ultrasound guidance, and cannulation of peripheral venous lines. However, catheter placement entails inherent risks and potential complications, depending on the chosen venipuncture site, technique, and catheter type (9,10). Immunocompromised pediatric oncology patients are particularly susceptible to complications (6).
Complications may arise during the catheter application, immediately afterward, or after a certain period, allowing for classification as early (≤30 days) or delayed (>30 days).
Complications can also be categorized based on their severity, specifically as minor and major. Minor complications typically do not necessitate surgical intervention or specific medical therapy for a period shorter than 24 hours. On the other hand, major complications encompass issues that demand early surgical intervention or extended medical treatment requiring a hospital stay exceeding 24 hours, with the potential for life-threatening outcomes.
Problems may arise during the placement of the central venous catheter, often involving injury to surrounding vital structures or incorrect positioning of the catheter tip. Early complications commonly include cardiac arrhythmia (23% – 25%), accidental arterial puncture (0% – 15%), hemothorax (0.1% – 11%), pneumothorax (1% – 4%), and air embolism (11-14). Delayed complications in central catheter placement often involve migration, mechanical issues (9%), complications related to the catheter material (2%) (13,14), infections (15), thrombosis (50%) (16), and fibrin pooling in the catheter.
Traumatic, infectious, or thrombotic complications of this nature can pose life-threatening risks, lead to extended hospitalization, and undoubtedly incur additional costs for prolonged treatment (6,17-19).
In such instances, a non-functional central venous line is simply extracted, necessitating the placement of a new central venous catheter (20).
The dislocation of the catheter in a central venous catheter represents a commonly encountered delayed complication. This complication occurs after the initial placement of the catheter and involves the movement or displacement of the catheter from its intended position within the vascular system. Unlike some early complications that manifest during or shortly after the insertion procedure, dislocation tends to become apparent over time (20-23).
The dislocation of the catheter can result in its tip being improperly positioned, potentially leading to issues such as inadequate delivery of medications or therapies, as well as posing risks of damage to surrounding structures. Detection of catheter dislocation often involves diagnostic imaging techniques, such as X-rays, to ascertain the catheter’s current location within the vascular system.
Managing catheter dislocation typically involves repositioning the catheter through appropriate medical interventions. Additionally, measures are taken to secure the catheter in its revised position to minimize the likelihood of further dislocation (21).
Given the importance of central venous catheters in the administration of various medical treatments, prompt identification and resolution of catheter dislocation are essential to maintain the efficacy and safety of ongoing patient care. Regular monitoring and follow-up assessments are crucial to identify and address any complications, including dislocation, in a timely manner, thereby optimizing the functionality and longevity of the central venous catheter.
Catheter occlusion in a central venous catheter is a prevalent delayed complication that can impact the functionality of the catheter over time. This complication occurs after the initial placement of the catheter and involves the blockage or obstruction of the catheter lumen, hindering the normal flow of fluids or medications through the catheter (22,23).
The occlusion of the central venous catheter can result from various factors, including the accumulation of blood clots, fibrin deposits, or precipitates of medications within the catheter lumen. Additionally, the formation of a thrombus around the catheter tip or within the blood vessels can contribute to occlusion.
Symptoms of catheter occlusion may include difficulty in aspirating blood or infusing fluids, changes in pressure readings during catheter use, or a noticeable decrease in the effectiveness of therapies administered through the catheter. Diagnostic measures, such as catheter patency checks and imaging studies, may be employed to identify and confirm the occlusion.
Addressing catheter occlusion often involves interventions to restore catheter patency. Healthcare professionals may use techniques such as instilling a thrombolytic agent or saline solution into the catheter, applying gentle catheter flushing, or employing mechanical devices designed to break down obstructions. In some cases, catheter replacement may be necessary if occlusion persists despite initial interventions (6,19,21).
Preventive measures include regular flushing of the catheter with appropriate solutions, adherence to prescribed flushing protocols, and ensuring proper care and maintenance of the catheter. Routine monitoring and assessment of the central venous catheter play a crucial role in early detection and management of occlusion, helping to sustain the catheter’s effectiveness and minimize potential complications for patients requiring long-term intravascular access.
Catheter-related thrombosis in a central venous catheter represents a common delayed complication that can significantly impact the vascular access and overall well-being of the patient. This complication occurs subsequent to the initial catheter placement and involves the formation of blood clots within or around the catheter, obstructing the normal blood flow through the vessel.
The development of thrombosis in the central venous catheter is often multifactorial, influenced by factors such as prolonged catheter dwell time, hypercoagulability of the patient, and catheter-related trauma to the vessel wall. Thrombosis can manifest in various forms, including the formation of clots within the catheter lumen, around the catheter tip, or extending into the larger blood vessels (22,23).
Patients with catheter-related thrombosis may experience symptoms such as swelling, pain, or discoloration at the catheter site, as well as difficulty in aspirating blood or infusing fluids through the catheter. Diagnostic measures, including imaging studies such as ultrasound or venography, are often employed to confirm the presence and extent of thrombosis (20-25).
Managing catheter-related thrombosis involves a combination of anticoagulation therapy and interventions to address the underlying causes. Anticoagulant medications may be administered to prevent the extension of the clot and reduce the risk of further complications. In some cases, the removal or replacement of the catheter may be necessary to eliminate the source of thrombosis (20-26).
Preventive measures to reduce the risk of catheter-related thrombosis include regular assessment of catheter function, adherence to anticoagulation protocols in high-risk patients, and ensuring proper catheter placement techniques. Healthcare professionals play a crucial role in monitoring and promptly addressing any signs of thrombosis, contributing to the maintenance of vascular access and the overall safety of patients relying on central venous catheters for medical treatment (26,27).
Aim of the study:
The main goal of this study is to assess the frequency of delayed complications in patients utilizing two distinct types of long-term central venous catheters. The first type involves tunneling, specifically the Hickman-monolumen and Broviac-multilumen catheters. The second type comprises totally implanted long-term central venous catheters, specifically the Bard-port. The study aims to provide valuable insights into the incidence of complications that occur beyond the initial placement period, offering a comprehensive understanding of the safety and efficacy profiles of these two catheter types in clinical practice.
Material and methods
Our study is prospective, interventional clinical study performed on 120 hemato-oncology pediatric patients scheduled for chemotherapy using a long-term central venous catheter and conducted in the period from January 2021 to January 2023.
The study was performed at the University Clinic for Traumatology, Orthopedics, Anesthesia with Resuscitation and Intensive Care and Emergency Center (UC TOARILUC – KARIL) in collaboration with the University Clinic for Pediatric Surgery and the Clinic for Children’s Diseases.
The study included pediatric patients who met the specified inclusion criteria, while those exhibiting factors for exclusion were not considered for participation. Enrolled participants comprised children with various conditions, including acute lymphoblastic leukemia, myeloid leukemia, non-Hodgkin and Hodgkin lymphoma, as well as solid tumors such as CNS tumors, rhabdomyosarcoma, Wilms’ tumor, and bone tumors. The study encompassed individuals aged 2 to 14 years, with a body mass index (BMI) below 25 kg/m2, falling within American Association of Anesthesiologists (ASA) classes I to III. Inclusion criteria also specified patients with normal infectious markers, neutrophil and platelet counts, appropriate hemostasis, and those whose parents or guardians provided informed written consent for study participation.
Patients falling below the age of 2 or surpassing 14 years, those with a BMI exceeding 25 kg/m2, individuals with ongoing or suspected infections previously treated with broad-spectrum antibiotics, patients exhibiting elevated infectious parameters, those with hemostatic disorders, thrombocytopenia, neutropenia, anemia (Hb < 100g/l), individuals allergic to heparin, and pediatric patients with kidney or liver diseases were systematically excluded from the study.
The study involved the randomization of patients into two equally sized groups. The first group consisted of 60 pediatric oncology patients who underwent the application of a totally implanted long-term central venous catheter under general endotracheal anesthesia. The second group comprised 60 pediatric oncology patients who underwent the application of an external tunneling long-term central venous catheter under general endotracheal anesthesia.
Following collaborative discussions with colleagues from the Clinic for Children’s Diseases and pre-operative preparations, a designated date for central venous catheter placement was established. One day before the procedure, patients underwent a thorough examination and anesthesia assessment at the UC TOARILUC anesthesiology outpatient clinic.
Upon admission to the Clinic for Pediatric Surgery, the invasive procedure commenced. Standard non-invasive hemodynamic monitoring, including ECG, non-invasive blood pressure, and pulse oximetry, was conducted in the operating room for pediatric patients. Following the induction of inhalation anesthesia (Sevoflurane-O2), a peripheral venous line was established for the administration of muscle relaxants and opioid analgesics. Subsequently, after endotracheal intubation and ensuring adequate hemodynamic and respiratory monitoring, the central venous catheter placement procedure was initiated.
The patient was appropriately positioned, adopting the Trendelenburg position, and the operating field was meticulously prepared using aseptic techniques. An ultrasound technique, employing the Siemens Acuson P500 with a linear probe, was utilized to identify anatomical structures for port placement. The central venous catheter placement in both study groups followed Seldinger’s method, targeting the vena jugularis interna.
For patients in the first group, vena jugularis interna was punctured, securing venous access. A guide-wire was then inserted, through which a silicone dilator was threaded, ensuring its tip reached the venous blood vessel. Subsequently, a subcutaneous pocket was created along the middle clavicular line, 3-5 cm below the right clavicle. Following precise hemostasis, the silicone reservoir, connected to the silicone catheter, was positioned within the subcutaneous pocket. A subcutaneous tunneling procedure was employed to establish communication between the pocket and the vessel puncture site. The catheter was then passed through this subcutaneous tunnel, using a metal guide to reach the insertion site of the silicone dilator. The silicone catheter’s tip was inserted into the venous blood vessel via the placed silicone dilator, reaching vena cava superior through vena jugularis interna. The silicone catheter’s height was determined by measuring from the insertion site to the sternal angle between the manubrium and the body of the sternum. Closure of the subcutaneous pocket was achieved through surgical sutures. The fully implanted catheter’s position was verified by aspiration through the silicone reservoir, with a positive test indicating the return of venous blood. At the procedure’s conclusion, the catheter received a 2.5 ml injection of heparin solution (100 IU of heparin in 1 ml of physiological solution). The tip’s position was confirmed through a native X-ray of the lungs.
For patients in the second group, who underwent the application of an external tunneling long-term central venous catheter, the initial phase mirrored that of patients in the first group. Venous access was established by puncturing the vena jugularis interna, and a guide wire was introduced, subsequently removing the metal cannula from the blood vessel. The silicone dilator, serving as a guide with its tip positioned in the venous blood vessel, was then threaded over the guide wire. A small subcutaneous incision of 1 cm was made in the middle clavicular line, 3-5 cm below the clavicle, after achieving adequate hemostasis. Following this, a subcutaneous tunneling procedure was performed from the surgical incision site to the insertion point of the silicone dilator.
Through the created subcutaneous tunnel, the silicone catheter was threaded from the surgical incision site to the positioned dilator with the aid of a plastic guide. Utilizing the guide dilator, the tunneling central venous catheter was introduced into the venous blood vessel, with its tip placed in v. cava superior. The outer portion of the catheter was secured with a surgical suture to the surgical incision. The tunneling central venous catheter featured a safety cuff (Surecuff-Tissue Ingrowth cuff) positioned in the subcutaneous tunnel 1-2 cm from the surgical incision. This safety cuff served to anchor the catheter to the surrounding subcutaneous tissue while acting as a physical barrier against the transmission of microorganisms. Procedures for verifying the position and height of the catheter aligned with those conducted for patients in the first group.
Following the interventional procedure and awakening from general anesthesia, patients from both groups underwent monitoring in the recovery room for one hour, where potential early complications were observed. After concluding the recovery room stay and undergoing X-ray diagnostics, patients were transferred to the Children’s Disease Clinic for monitoring late complications arising from the invasive procedure. The initiation of prescribed chemotherapy could commence on the same day.
Results
The results obtained by processing and analyzing data from 120 subjects, hemato-oncology pediatric patients who are to be administered chemotherapy using a long-term central venous catheter, are shown.
The gender structure of the respondents consisted of 68 male patients (56.67%) and 52 female patients (43.33%).
Patients ranged in age from 2 to 14 years, with a mean age of 6.1 ± 3.5 years. Half of the patients were older than 5 years (median=5 years).
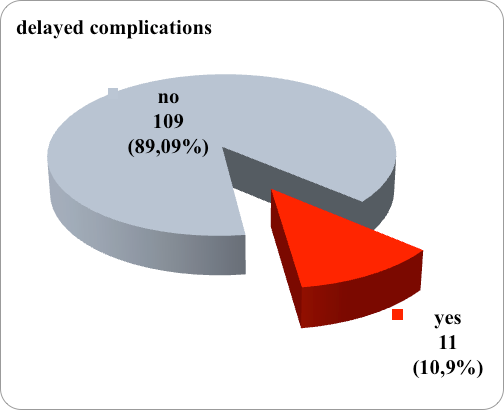
Delayed complications after the application of a central venous catheter were registered in 14 patients, that is, the incidence of delayed complications was 11.67%. (Figure 1)
Figure 1. Graphic representation of frequency of delayed complications
Regarding the type of late complications, in 5 patients dislocation of the catheter was registered, in 2 catheter thrombosis, in 4 occlusion of the catheter. (table 1)
Delayed complications were manifested significantly more often by patients from the group with ETLCVC compared to patients from the group with TILCVC – 9 vs 2.
Table 1. Frequency of delayed complications in both groups
| Delayed complications | n | group 1
n |
group 2
n |
| Yes | 11 | 9 | 2 |
| No | 109 | 51 | 58 |
Group 1 – ETLCVC (external tunneling long-term central venous catheter)
Group 2 – TILCVC (totally implanted long-term central venous catheter)
Catheter dislocation was recorded in 1 (1.67%) patients with TILCVC and 4 (6.67%) patients with ETLCVC. The higher prevalence of this complication in patients in whom ETLCVC was applied was not confirmed statistically as significant (p=0.17). (table 2)
Table 2. Frequency of catheter dislocation in both groups
| Dislocation of the catheter | n | group 1
n (%) |
group 2
n (%) |
p – value |
| Yes | 5 | 4 (6.67) | 1 (1.67) | X2=1.9
p=0.17 |
| No | 115 | 56 (93.33) | 59 (98.33) |
Group 1 – ETLCVC (external tunneling long-term central venous catheter)
Group 2 – TILCVC (totally implanted long-term central venous catheter)
X2(Pearson Chi-square test)
Catheter thrombosis complication occurred insignificantly more often in patients with ETLCVC application, that is, in 2 (3.33%) patients from this group, and in no patient from the TILCVC group (p=0.15). (table 3)
Table 3. Frequency of catheter thrombosis in both groups
| Thrombosis of the catheter | n | group 1
n (%) |
group 2
n (%) |
p – value |
| Yes | 2 | 2 (3.33) | 0 | X2=2.0
p=0.15 |
| No | 118 | 58 (96.67) | 60 (100) |
Group 1 – ETLCVC (external tunneling long-term central venous catheter)
Group 2 – TILCVC (totally implanted long-term central venous catheter)
X2(Pearson Chi-square test)
Catheter occlusion was registered in 3(5%) patients with ETLCVC and 1(1.67%) patients with TILCVC, and without a statistically significant difference between the two groups (p=0.31). Catheter occlusion was insignificantly more common in patients with NTDCVK application. (table 4)
Table 4. Frequency of catheter occlusion in both groups
| Catheter occlusion | n | group 1
n (%) |
group 2
n (%) |
p – value |
| Yes | 4 | 3 (5) | 1 (1.67) | X2=1.0
p=0.31 |
| No | 116 | 57 (95) | 59 (98.33) |
Group 1 – ETLCVC (external tunneling long-term central venous catheter)
Group 2 – TILCVC (totally implanted long-term central venous catheter)
X2(Pearson Chi-square test)
Discussion
Long-term central venous catheters are frequently utilized for various medical purposes such as blood sample collection, intravenous hydration, and the administration of medications to patients with diverse pathologies. The initial descriptions of these catheters were provided by Broviac in 1973 (28) and later by Hickman in 1979 (29). These catheters were subcutaneously tunneled and featured a subcutaneous cuff. Totally implanted long-term central venous catheters (TILCVCs) offer several advantages, including the absence of external dressings, enhanced patient mobility, and the need for monthly injections of heparin solution for maintenance. Consequently, TILCVCs are associated with fewer infections and complications compared to externally tunneled catheters (30). The overall complication rate for long-term central venous catheters is estimated to be between 10% and 15% (20,29). This paper focuses on delayed complications (such as catheter dislocation, catheter thrombosis and catheter occlusio) and their correlation with the type of catheter applied (23, 24, 26).
The study encompassed 120 pediatric oncology patients aged 2-14 years, with 90 patients diagnosed with leukemia (75%), 22 with solid tumors (18.3%), and 8 with lymphoma (6.7%). Acute lymphoblastic leukemia accounted for the majority of diagnoses (44%), followed by lymphoid leukemia (22.5%). The patients were categorized into two groups based on the type of long-term central venous catheter used for cytostatic therapy. Both groups exhibited homogeneity in terms of gender and age, with a predominant male gender in the first group (55%) and the second group (58%). The average age in both groups was 6.1 ± 3.5 years.
In this study, the overall rate of delayed complications was 10.9%. Long-term central venous catheters (CVCs) are indispensable tools in the management of pediatric oncology cases, providing reliable vascular access for the administration of chemotherapy, blood products, and other essential medications. However, these catheters are not without complications, and three significant issues warrant discussion: catheter dislocation, catheter thrombosis, and catheter occlusion.
Catheter dislocation, or unintended movement from its original placement site, poses a risk to the effective delivery of medical treatments. In pediatric oncology, the dynamic nature of a child’s growth and development can contribute to catheter dislocation. Additionally, physical activity and inadvertent tugging on the catheter may further predispose pediatric patients to this complication. Preventive strategies, such as securement techniques, should be implemented to minimize the risk of dislocation. Routine assessments and vigilant monitoring play a crucial role in the early identification of dislocation, allowing for timely intervention and the prevention of treatment interruptions(12,16,22,29).
Catheter thrombosis, the formation of blood clots within the catheter lumen or the surrounding vasculature, is a frequent concern in long-term CVC use. Pediatric oncology patients, often undergoing aggressive chemotherapy regimens, are at an increased risk of thrombotic events. Thrombosis can impede blood flow, leading to catheter malfunction and potentially compromising the delivery of therapies. Prophylactic measures, including appropriate anticoagulation strategies and routine flushing protocols, are essential to minimize the risk of thrombosis. Regular monitoring for signs of thrombosis, such as sluggish blood return or resistance during flushing, enables prompt detection and intervention(16,27,29).
Catheter occlusion, the blockage of the catheter lumen, is another common complication in long-term CVC use. In pediatric oncology cases, occlusion may result from the accumulation of blood products, drug precipitates, or fibrin within the catheter. Routine flushing with appropriate solutions, maintaining adequate hydration, and employing proper catheter care practices can mitigate the risk of occlusion. Regular assessment of catheter patency and prompt intervention, such as catheter clearance protocols, are crucial in managing occlusive events and ensuring uninterrupted therapy delivery(26).
In addressing these complications collectively, a comprehensive and multidisciplinary approach is paramount. Healthcare providers in pediatric oncology must remain vigilant in adopting preventive measures, conducting routine assessments, and promptly addressing complications to optimize the safety and efficacy of long-term CVC placement. Moreover, continuous education for healthcare teams and caregivers is essential to enhance awareness and promote adherence to best practices, ultimately improving the overall care and outcomes for pediatric oncology patients reliant on long-term central venous catheters.
Conclusion
The placement of long-term central venous catheters (CVCs) in pediatric oncology cases is a crucial component of comprehensive medical care, providing essential vascular access for the administration of critical therapies. Despite the undeniable benefits of CVCs in enhancing patient comfort and treatment efficacy, healthcare providers must navigate potential complications that may arise during their prolonged use.
Catheter dislocation, thrombosis, and occlusion emerge as notable challenges in the management of long-term CVCs in pediatric oncology. The dynamic nature of pediatric patients’ growth and development, coupled with the intensive medical interventions they undergo, necessitates a nuanced and proactive approach to address these concerns.
Implementing preventive strategies, such as securement techniques to minimize dislocation risks, anticoagulation protocols to mitigate thrombotic events, and vigilant catheter care practices to prevent occlusions, is paramount. Regular assessments, monitoring, and prompt intervention are crucial components of a comprehensive care plan aimed at optimizing the safety and efficacy of long-term CVC placement.
Moreover, healthcare providers must engage in ongoing education and training to stay abreast of the latest advancements in catheter placement techniques, complication management, and evidence-based practices. Collaborative efforts involving multidisciplinary teams, including pediatric oncologists, nurses, and caregivers, are essential to ensure a holistic and patient-centered approach to long-term CVC care.
While challenges may arise, the benefits of long-term CVCs in pediatric oncology, including improved treatment delivery and reduced procedural stress, underscore their indispensable role in the continuum of care. As technology and medical knowledge advance, ongoing research and refinement of protocols will further enhance the safety and effectiveness of long-term CVC use in pediatric oncology cases.
References
- World Health Organization. Cure All framework: WHO global initiative for childhood cancer: increasing access, advancing quality, saving lives. World Health Organization 2021. DOI: https://apps.who.int/iris/handle/10665/347370.
- Kowalczyk JR, Samardakiewicz M, et al. European Survey on Standards of Care in paediatric oncology centres. Eur. J. Cancer 2016;61:11-19.
- Orgel E, Ji L, Pastor W. Infectious morbidity by catheter type in neutropenic children with cancer. Pediatr. Infect. Dis 2014;33(3):263-266.
- Santana FG, Moreira-Dias PL. Central Catheter of Peripheral Insertion in Pediatric Oncology: a Retrospective Study. Revista Brasileira de Cancerologia 2018;64(3):339-345.
- Fadoo Z, Nisar MI, Iftikhar, R, et al. Peripherally Inserted Central Venous Catheters in Pediatric Hematology/Oncology Patients in Tertiary Care Setting: A Developing Country Experience. J. Pediatr. Hematol. Oncol. 2015;37(7):421-423.
- Anttilla VJ. Central venous catheter care for children with cancer should focus on early infections. Acta Paediatrica 2018;108(2): 204-205.
- Revel-Vilk S, Kenet G, et al. Risk factors for central venous catheter thrombotic complications in children and adolescents with cancer. Cancer 2010; 116:197-205.
- Petry J, Rocha KT, Madalosso AR, Carvalho RM, Scariot M. Cateter venoso central de inserção periférica: limites e possibilidades. Rev Eletr Enf. 2012;14(4):937-943.
- Di Santo MK, Takemoto D, Nascimento RG, et al. Cateteres venosos centrais de inserção periférica: alternativa ou primeira escolha em acesso vascular? J. Vas.c Bras. 2017;16(2): 104-112.
- Crocoli A, Tornesello A, et al. Central venous access devices in pediatric malignancies: a position paper of Italian Association of Pediatric Hematology and Oncology. J. Vasc. Access. 2015; 16(2):130-136.
- Di Carlo I, Biffi R, Niederhuber JE. Totally implantable venous access devices: Management in mid- and long-term clinical setting. Springer-Verlag Italia 2012. DOI: https://doi.org/10.1007/978-88-470-2373-4
- van den Bosch CH, Spijkerman Ј, et al. Central venous catheter–associated complications in pediatric patients diagnosed with Hodgkin lymphoma: implications for catheter choice. Support Care Cancer 2022;30(10): 8069-8079.
- Biagi E, Arrigo C, Dell’Orto MG, et al. Mechanical and infective central venous catheter-related complications: a prospective non-randomized study using Hickman and Groshong catheters in children with haematological malignancies. Support Care Cancer 1997;5: 228-233.
- Fratino G, Mazzola C, Buffa P, et al. Mechanical complications related to indwelling central venous catheter in pediatric hematology/oncology patients. Pediatr. Hematol. Oncol. 2001; 18: 317-324.
- Mermel LA, Farr BM, Sherertz RJ, et al. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 2001; 32: 1249-1272.
- Journeycake JM, Buchanan GR. Thrombotic complications of central venous catheters in children. Curr. Opin. Hemato. 2003; 10: 369-374.
- Giordano P, Saracco P, Grassi M, et al. Recommendations for the use of long-term central venous catheter (CVC) in children with hemato – oncological disorders: management of CVC-related occlusion and CVC- related thrombosis. On behalf of the coagulation defects working group and the supportive therapy working group of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Ann. Hematol. 2015;94(11): 1765-1776.
- Raad I, Chaftari AM. Advances in prevention and management of central lineassociated bloodstream infections in patients with cancer. Clin. Infect. Dis. 2014;59(5): 340-343.
- Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection 2015; 43: 29-36.
- Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications
of Central Venous Access Devices: A Systematic Review. Pediatrics 2015; 136(5): 1331-1344. - Kusminsky RE. Complications of central venous catheterization. J. Am. Coll. Surg. 2007; 204(4): 681-696.
- Tsotsolis N, Tsirgogiann K.et al. Pneumothorax as a complication of central venous catheter insertion. Ann. Transl. Med. 2015;3(3): 40.
- Orci LA, Meier RP, et al. Systematic review and metaanalysis of percutaneous subclavian vein puncture versus surgical venous cutdown for the insertion of a totally implantable venous access device. Br. J. Surg. 2014; 101(2): 8-16.
- Di Carlo I, Pulvirenti E, et al. Increased use of percutaneous technique for totally implantable venous access devices. Is it real progress? A 27-year comprehensive review on early complications. Ann. Surg. Oncol. 2010; 17(6): 1649-1656.
- Nazinitsky A, Covington M, Littmann L. Sinus arrest and asystole caused by a peripherally inserted central catheter. Ann. Noninvasive Electrocardiol. 2014; 19(4): 391-394.
- Gibson F, Bodenham A. Misplaced central venous catheters: Applied anatomy and practical management. Br. J. Anaesth. 2013;110(3): 333‑346.
- Mastrandrea G, Giuliani R, Graps EA. International good practices on central venous catheters’ placement and daily management in adults and on educational interventions addressed to healthcare professionals or awake/outpatients. Results of a scoping review compared with the existent Italian good practices. Front Med (Lausanne). 2022 Oct 6;9:943164. doi: 10.3389/fmed.2022.943164.
- Wurzel CL, Halom K, Feldman JG, Rubin LG. Infection Rates of Broviac-Hickman Catheters and Implantable Venous Devices. Am J Dis Child. 1988;142(5):536–540. doi:10.1001/archpedi.1988.02150050074036
- Higgins JPT. Cochraneance Handbook for Systematic Reviews of Interventions Version 5.1.0[EB/OL]. The Cochrane Collaboration (2011). Available online at: https://training.cochrane.org/handbook/archive/v5.1.