UDK: 612.115.1:616-005.6
Nancheva J1, Kiprijanovska B1, Nancheva Bogoevska A2, Naumovski F1
1University Clinic for Traumatology, Orthopedic Diseases, Anesthesia, Reanimation, Intensive Care and Emergency Centre, Medical Faculty, University “Ss. Cyril and Methodius,” Skopje, Macedonia
2City General Hospital “8-mi Septemvri”, Department of Radiology
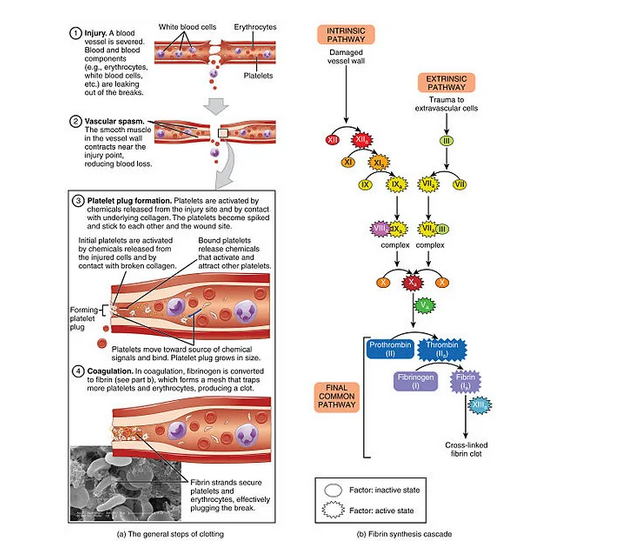
Hemostasis can be defined as a physiological process that stops bleeding after injury of blood vessels. It is a complex and highly regulated process to localize the blood clot only to the site of injury. The hemostatic system in the human body is based on the components of Virchow’s triad:
- vascular injury,
2. change in blood coagulability,3. disturbance of blood flow (stasis). If the third component (blood flow) is excluded, hemostasis can be defined as an inter-reaction between the blood vessel wall, blood cell components and plasma proteins that maintain the hemostatic balance. The final outcome of hemostasis is coagulation of blood at the site of vascular injury(1,3). Hemostasis can be divided into primary, secondary and tertiary hemostasis. These three independent mechanisms combine to maintain hemostatic balance. Before primary hemostasis begins at the site of blood vessel injury, a local contraction occurs in order to reduce blood loss from the site of the damaged blood vessel. It is the result of nerve reflexes and local myogenic spasm, most likely caused by the painful impulse at the site of blood vessel injury and surrounding tissue. This spasm of the damaged blood vessel extends several centimeters along it. The greater damage of blood vessel produces the greater contraction of the blood vessel. When a blood vessel is cut sharply, it bleeds more than in crushed tissue. The local spasm of the damaged blood vessel can last several minutes or even hours. It has been shown that in patients with bruised lower legs, there is a strong vasoconstriction of the large blood vessels of the lower legs, such as the anterior tibial artery, as a compensatory mechanism to prevent lethal blood loss (1,2,3).
Primary hemostasis is a process aimed at the formation of a platelet plug, that plugs the site of injury, through the cellular interaction of platelets with the sub-endothelium of the injured blood vessel. Small injuries to blood vessels that occur every day are closed with platelet plugs, and larger injuries to blood vessels with blood coagulum (plugs). Platelets together with erythrocytes and leukocytes are the cellular part of the blood. Platelets are created by the megakaryocytes in the bone marrow, more precisely as the buds of the megakaryocyte are separated from it and released into the blood. The platelet does not have a nucleus, but its cytoplasm has:
1.Actin and myosin molecules that enable the contraction of Tr. 2. Remains of endoplasmic reticulum and Golgi apparatus that synthesize various enzymes and store a large amount of calcium.3. Enzymatic systems that synthesize prostaglandins. 4. Protein-factor of fibrin stabilization.5. Growth factor that enables the growth of vascular endothelial cells, vascular smooth muscle cells and fibroblasts.6. Glycoprotein in the cell wall of the Tr. which enables the adhesion of the Tr. to the endothelial cells of the blood vessel and the collagen from the deep parts of the blood vessel. 7. Phospholipid – which can activate the “intrinsic” system. 8. Protein enzyme adenyl-cyclase stimulates the creation of C-AMP (enables platelet activity). 9. The half-life of Tr is 8-12 days. It is degraded by macrophages in the spleen.10. Thrombus takes an irregular shape and sticks to the collagen from the endothelium of the damaged part of the blood vessel, and at the same time it secretes thromboxane A, which increases the adhesion of other thrombin to the platelet plug. The platelet plug does not close the lumen of the blood vessel, but the blood coagulum can close it (1,2,3,4,5,6). Secondary hemostasis depends on the activation of coagulation proteins sequentially, which is regulated by many feedback mechanisms. The formation of a coagulation plug – coagulum at the place of the damaged part of the blood vessel begins, and it is created in 15 to 20 seconds if the injury to the blood vessel is large, and 1-2 minutes if the injury to the blood vessel is small. If the opening of the blood vessel is not too large, in 3-6 minutes the coagulum fills the cut part, and in 30-60 minutes it retracts and can almost close the blood vessel. In the created blood coagulum, fibroblast threads penetrate, which make a fibroblast network in which Tr, blood cells, plasma are trapped, so that later this structure can be organized and passed into connective tissue (7-10 days), i.e., complete healing of the blood vessel. When it comes to heavy bleeding, a hematoma is created in the tissues, which itself produced enzymes that break it down (2,3,4,5,6). Stages of Blood Coagulation In the blood and tissues there are about 40 substances that act on blood clotting, namely substances that enable blood pro-coagulation and substances that inhibit blood anticoagulation. Basically, these two systems are in balance, but with greater dominance of anticoagulation. But when a blood vessel is injured, pro-coagulation prevails over anticoagulation and the formation of a coagulum occurs.
The first stage of blood coagulation, when a blood vessel breaks or when blood is damaged, is to create a prothrombin activator (substance complex).At the second stage, the prothrombin activator enables the conversion of prothrombin into thrombin.In the third phase, thrombin acts as an enzyme and converts fibrinogen in the coagulum into fibrin threads (in which Tr, blood cells and plasma are trapped) in a period of 10 to 15 seconds from the injury of the blood vessel (3,4,5,6). The Conversion of Prothrombin to Thrombin When a blood vessel is injured, a prothrombin activator is immediately created, which will allow prothrombin to pass into thrombin, and thrombin to lead to the polymerization of fibrinogen molecules into a fibrin network. Prothrombin (factor II) is a plasma protein, alpha 2 – globulin with a concentration of 0.15g/l, with a molecular mass of 68,700. It is created in the liver, and its reserves in the liver are used up in 24 hours if no new ones are created. It is unstable and breaks down into smaller parts – thrombin, which has molecular weight of 33,700. Vitamin K is needed for synthesis of prothrombin – (f-or II) in the liver, as well as for factor VII, IX, X, protein C and protein S. Deficiency of vitamin K and the existence of liver disease, obstruction of bile ducts, increase the possibility of bleeding, because Vit.K is created in the intestines by intestinal bacteria or comes from food and is resorbed from the intestines with the help of fats and bilirubin. In such patients, 4-8 hours Vit. K is given preoperatively. So, if there are even the smallest number of intact hepatocytes, coagulation factors (VII, IX, X) and prothrombin can be created.
Updated Classification of Vitamin K-Dependent Clotting Factors or Protein Defects:
The Conversion of Fibrinogen to Fibrin Fibrinogen is a plasma protein with a large molecular weight of 340,000 in a concentration of 1-7g/l. It is synthesized in the liver, so as for prothrombin, liver diseases reduce its synthesis. The large molecular mass prevents it from leaving the blood bed, but with pathologically increased capillary permeability, it exits into the interstitium and can lead to coagulation of the interstitial fluid. Fibrinogen in the presence of thrombin, which is a protein enzyme with proteolytic properties, allows fibrinogen to be broken down into fibrin threads that form a fibrin network and solidify the blood coagulum, which is still unstable at the beginning, but under the action of a fibrin stabilization factor (which is secreted mostly from the Platelets) becomes firmer. In that fibrin network there is Tr, Er, Le and plasma. After a few minutes of the formation of the blood coagulum, it begins to retract (30-60minutes) and serum that does not contain fibrinogen and most of the coagulation factors comes out of it. To retrace it, platelets are also needed, from which the pro-coagulation substances are released (1,3,5,6). Generation of Prothrombin Activator The creation of prothrombin activator is by two mechanisms: an extrinsic mechanism and an intrinsic mechanism of blood coagulation. |
An extrinsic mechanism of blood clotting begins with the generation of prothrombin activator when blood comes into contact with a damaged vessel wall or with tissue outside the vessel and tissue thromboplastin is released. This mechanism includes three stages: 1. Release of tissue thromboplastin (factor III) from the damaged tissue. 2. Activation of factor X to create activated factor X, through coupling of tissue glycoprotein with factor VII-proconvertin which act enzymatically on factor X. 3. Activated factor X in the presence of Ca++, factor V (proaccelerin) creates the activated prothrombin which allows prothrombin in the presence of Ca++ (factor IV) to pass into thrombin.
Table1.Intrinsic and extrinsic mechanism of blood clotting. An internal mechanism of blood clotting begins with the creation of a prothrombin activator when the blood, that is, factor XII and Tr come into contact with the collagen from the damaged blood vessel wall. This mechanism includes:
- Activation of factor XII (Haegeman factor) and release of platelet phospholipids.
- Activation of factor XI by activated factor XII which acts as an enzyme.
- Activation of factor IX by activated factor XI acting as an enzyme.
- Activation of factor X by factor VIII, factor IX and Ca ++.
- Activated factor X together with factor V and Ca ++ initiate the prothrombin activator which allows prothrombin to pass into thrombin, and thrombin to act as an enzyme in the reaction for fibrinogen to pass into fibrin threads.
6. The role of Ca ++ in all cascade processes of the internal and external coagulation mechanism. The internal mechanism of coagulation is much slower than the external one. It takes 2-6 minutes to cause coagulation (3,5,6). Tertiary hemostasis describes fibrinolysis, namely the lysis of the blood clot that was created during damage to the blood vessel wall and thereby restores the normal integrity of the blood vessel. Fibrinolysis occurs under the action of fibrinolysin (plasmin), which is a proteolytic enzyme derived from profibrinolysin, which is found in the plasma as euglobulin which when activated passes into plasmin (fibrinolysin). It is actually found in the blood coagulum as plasminogen together with other plasma proteins. It will not turn into fibrinolysin (plasmin) if it is not activated with the help of 1. thrombin, 2. activated factor XII, 3. lysosomal enzymes from the damaged tissue, 4. factors from the blood vessel endothelium. Activated fibrinolysin begins to act 1-2 days after the formation of the blood coagulum in order to lyse the fibrin threads, factor V, VIII, XII and prothrombin from it. Natural activators of fibrinolysin are urokinase, streptokinase, and its natural inhibitor is alpha 2-antiplasmin(3,4,5,6). What is the Importance of the Fibrinolytic System? The fibrinolytic system enables dissolution of blood clots and the cleaning of blood that has come out of the tissues from the damaged blood vessels. The coagulum in the small blood vessels is more easily fibrinolyzed and allows the patency of the blood vessels. Larger coagulum of large blood vessels is more difficult to lyse and the blood vessel remains most often obstructed.Hemostasis is a complex mechanism depending on a very delicate balance of hemostasis and pro-coagulant and anticoagulant factors. These factors are essential for maintaining the fluidity of blood in intact blood vessels and promoting effective blood clotting in vascular injury. They are also essential for limiting clotting at the site of injury and avoiding its spread to other uninjured parts of the blood vessels(3,4,5,6). Evaluation of Hemostasis in Neonates and Children Hemostatic balance is different in newborns and children compared to adults, that is, hemostasis is in continuous evolution. Maturation begins early intra-uterine, until complete maturation for some factors and puberty.The neonatal hemostatic system is remarkably different from that of adults.Among other differences, neonates show hyporeactive platelets and decreased levels of coagulation factors, which translate into prolonged coagulation times (PT and PTT). Because preterm infants have a high incidence of bleeding, especially intraventricular hemorrhage, neonatologists often administer blood products (i.e., platelets and FFP) to nonbleeding infants with low platelet counts or prolonged coagulation times in an attempt to overcome these “deficiencies”and reduce the risk of bleeding. However, it is becoming increasingly clear that both platelet hyporeactivity and decreased levels of coagulation factors are effectively offset by other factors in neonatal blood that promote hemostasis (i.e., high vWF levels, high hematocrit and MCV, decreased levels ofnatural anticoagulants), resulting in a well-balanced neonatal hemostatic system, perhaps slightly biased towards a prothrombotic phenotype. Although life-saving in the presence of active major bleeding, administration of platelets and/or FFP to nonbleeding neonates (based on laboratory tests) not only failed to reduce bleeding, but was associated with increased neonatal morbidity and mortality, especially when platelets are given. Such evaluation of hemostasis is especially important in the first few months of postnatal life.At birth, plasma coagulation proteins have been shown to be about half of adult values. They are even lower in premature babies than in full-term newborns and increase postnatally, so that after a few months they reach the values of coagulation proteins as in adults, and for some coagulation factors, even until puberty(7,8,9,10).
Developmental Hemostasis
Children cannot be considered miniature adults in terms of hemostatic balance. There is a big difference between pediatric and adult hemostatic systems. In the 1980s, Maureen Andrews introduced the term “developmental hemostasis” to describe the changes that occur in the coagulation system, progressively, from the beginning of intra-uterine fetal life, postnatal, pediatric life to adulthood, and then in geriatric systems (8,10). Maternal coagulation factors do not cross the placenta, and the fetus begins the synthesis of coagulation proteins from the fifth week of intrauterine life, its blood can coagulate as early as 11 weeks of pregnancy. At gestational age between 19 – 23, reference values for coagulation factors are ten to thirty percent of adult values, progressively increasing to levels between 10% – 50% at gestational age between 30-38 weeks. The importance of hemostasis development is to prevent misdiagnosis and treatment of hemostatic problems in infants and children, and to explain the pathophysiological basis of hemorrhagic and thrombotic complications of all ages (1,2,8,9,10,11).
Difficulties in Interpreting the Hemostatic System in Infants and Children
There are limited data on the physiology of hemostasis in neonates and children when compared to the available data on the hemostatic system in adults. Many factors increase this limitation in neonates and children, since more reference ranges are needed that go with the age-related evolution of the hemostatic system, which in turn requires many patients to establish normative data, despite the difficulty of blood sampling in pediatric age groups(11,12).
Sampling Problems
Sampling in neonates and young infants relative to adults is a problematic process, which is a key point to consider. Heparin-contaminated blood samples are often obtained from central vascular catheters or pre-used heparinized syringes. All of these give incorrect results. Moreover, neonatal polycythemia (which is common), during collection of blood in the tube can affect the result due to the influence of the citrate-to-blood ratio (9:1) (11,12,13).
Defining Reference Values
Lack of use of appropriate reference values remains the most common obstacle in the interpretation of pediatric coagulation tests. One problem in defining age-dependent reference values is that functional levels of coagulation proteins change with the age of children. These changes are reflected in the results of some coagulation tests, such as activated partial thromboplastin time (APTT). Other tests are less affected by age-related changes in hemostatic factors, such as thromboelastography. Use of the international normalized ratio (INR) minimizes variation in prothrombin time (PT) results. Thrombin time in newborns is prolonged due to the absence of calcium during its derivation, as fibrinogen is present in a “fetal form” at birth, and to polymerize, it needs calcium. Another problem is that reference values are age-dependent and are dependent on each analyzer-reagent in many clinical laboratories. The same happens with aPTT interpretation. Many laboratories with their own reference values can lead to unnecessary referrals to hematologists, multiple searches, wrong diagnoses, cancellations of operations or excessive treatments of the child. Accordingly, ISTH, “The Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis” (2012) recommended to define, depending on age, reference values for each laboratory according to their technical conditions (13,14,15).
New Diagnostic Tests
Knowing the basics of developmental hemostasis in children and the large number of pediatric clinical studies, there was a need to introduce a new Endogenous Thrombin potential (ETP) analysis, which measures, in vitro, the overall ability of the hemostatic system to generate thrombin (16).
Age-related Changes in the Hemostatic System
At birth, there is a transient increase in the number of platelets, so that by the end of the first year it reaches the values as in adults (20). Although platelet functions are depressed in the neonatal period, bleeding time and platelet clotting time (PFA-100) were found to be paradoxically shortened in neonates by the end of the first month of life. Von Willebrand factor (factor 8) in newborns has been shown to be elevated and it decreases reaching adult levels after the first year (14,15,16,17).
Changes in Coagulation Proteins
Coagulation proteins in the fetus begin to be synthesized intrauterine in the 5th gestational week, and blood begins to coagulate from the 11th gestational week. Already, from 19-23 gestational weeks, the values of gestational coagulation factors are represented by 10-30% in relation to of adults, to progressively increase from 10-50% between 30-38 weeks of gestation. Gestational coagulation factors synthesized during fetal life are unable to cross the placenta, nor can maternal coagulation factors cross the placental barrier.
By the10th week of intrauterine life, coagulation proteins have measurable concentrations in plasma, gradually increasing with the progression of pregnancy. It has been shown in many studies that Vit. K-dependent factors: Prothrombin (FII), FVII, FIX and FX at birth have physiologically low values, below 30% of the adult values (while they do not have clinical manifestations of bleeding), especially they gradually increase to reach the adult level. The last to reach adult levels is FVII, which may not reach adult levels until age of 16. Levels of FXI, FXII and contact factors (pre-kallikrein and high-molecular-weight kininogen) gradually increase to adult values sometime around the 6th month after birth. Low values of contact factors are the cause of prolonged aPTT in the first months of life. A high basal metabolic rate in infants and children may accentuate the low level of plasma proteins by accelerating their clearance rate. Levels of fibrinogen, FV, FVIII, FXIII and von Willebrand- factor (vWF) are not decreased at birth. On the contrary, plasma levels of FVIII may rise and be higher than adult levels, requiring adjustment of the lower limit of normal. Also, vWF levels are elevated at birth and for the first 3 months of life. Regarding fibrinogen levels, not only is the fibrinogen is low at birth, but it has also been shown to exist in a “fetal form”, in cord blood. This “fetal form” of fibrinogen has a high sialic acid and phosphorus content relative to adult fibrinogen. Sialic acid directly in fibrinogen binds to Ca+2 leading to a reduction of repulsion between fibrinogen chains and facilitation of fibrin polymerization. Prolonged thrombin time (TT) in neonates may be attributed to different polymerization of fetal fibrinogen than adult fibrinogen, leading to the notion that infants have “dysfunctional fibrinogen” (1,7,8,9,10,11).
Natural Anticoagulants
- 1. Factors on the surface of the endothelium– The smooth surface of the endothelium (monomolecular layer of adsorbed proteins) of the blood vessels prevents the contact activation of F XII, Tr and contact proteins, and thus the chain of activation of the internal coagulation system.
- Thrombin is inhibited by many anticoagulants present in cord blood, such as antithrombin, 𝛼2-macroglobulin, dermatan sulfate as an anticoagulant and heparin cofactor II [38]. In neonates, 𝛼2-macroglobulin appears as a more potent inhibitor of thrombin than in adults, which accounts for the low levels of antithrombin in neonates. Despite the greater potency of neonatal 𝛼2-macroglobulin, thrombin inhibition is still slower in neonates than in adults (7).
- Fibrin threads that create the network of the coagulum act as anticoagulants, on the principle of binding thrombin to the very threads of the blood coagulum, and thus prevent the expansion of the coagulum.
- Antithrombin III is an alpha globulin that binds thrombin that remained unbound to fibrin threads and blocks the action of thrombin on fibrinogen for 12-20 minutes.It inactivates thrombin. In the first three months after birth, the concentration of antithrombin is 50% lower compared to adults who had recurrent thrombosis in heterozygous anti-thrombin deficiency. Its concentrations gradually increase during the first 6 months after birth. In neonates and infants, there are no data on thrombotic manifestations despite the low levels of antithrombin. 5. Heparin is a conjugated polysaccharide with a strong negative charge, by itself it has little or no anticoagulant effect, however, when bound to antithrombin III, it increases the anticoagulant power by a thousand times by removing thrombin. This antithrombin-heparin cofactor also removes from circulation the following coagulation factors XII, XI, IX, X. Heparin is secreted by basophilic cells in the blood and fat cells that are located pericapillary in the connective tissue of the entire organism, especially around the capillaries in the lungs and liver. The number of mast cells in these organs acts in some way protectively because in the venous slow current of blood come very small coagulum, and such coagulum from human antithrombin-heparin prevent the growth of these coagulum. Thrombin is inhibited by many anticoagulants present in cord blood, such as antithrombin, 𝛼2-macroglobulin, dermatan sulfate as an anticoagulant and heparin cofactor II (1,3,7).
Protein C, Protein S and Thrombomodulin
At birth, plasma concentrations of protein C and protein S are very low. Protein C levels remain low during the first 6 months of life. The low level of protein S is compensated with the increase of its functional activity. Protein S is present in an active form due to the absence of C4 binding protein in newborns. In addition, increased levels of 𝛼2-macroglobulin facilitate the interaction of protein S with activated protein C in the plasma of the newborn. Whereas plasma thrombomodulin levels increase in early childhood, and leveling off to adult-like values is reached in adolescence (1,2,7).
Conclusions
Understanding of developmental hemostasis is of crucial importance for achieving a diagnosis of bleeding and thrombotic disorders in infants and children, as well as avoiding hematological and non-hematological complications. Correct age-appropriate sampling techniques, specific reference to analyzers and reagents are crucial to avoid misdiagnosis and overtreatment. Finally, new assays should be developed, taking into account the basis of developmental hemostasis.
Points for Clinical Practice
- Proper sampling is a necessity in pediatric coagulation studies.Attention to details, repeatability of sampling, are all important to avoid erroneous results.
- Age-appropriate reference values are crucial for accurate diagnosis and treatment of coagulation disorders in infants and children, such as sudden changes in hemostatic protein concentrations during the first few months of life.
- Each laboratory should establish its own specific reference values depending on its technical conditions.
- Adult plasma products or drugs may have adverse effects on the neonate due to the multiple non-hemostatic functions of hemostatic proteins.
References:
- Monagle P, Ignjatovic V, Savoia H. Hemostasis in neonates and children: pitfalls and dilemmas. Blood Rev. 2010; 24(2):63-8. doi: 10.1016/j.blre.
- Monagle P, Massicotte P. Developmental haemostasis: secondary haemostasis . Fetal Neonatal Med. 2011; 16(6):294-300. doi: 10.1016/j.siny.2011.07.007.
- John E. Hall. Guyton and Hall Textbook of Medical Physiology. 14 th Edition,Saunders Elsevier, 2020,451-460.
- Difference Between Primary and Secondary Hemostasis-www.differencebetween.com,july, 2017.
- ”Primary hemostasis.” Khan Academy. N.p., n.d. Web. Available here (www.khanacademy.com), 28 June 2017.
- ”Secondary hemostasis.” Khan Academy. N.p., n.d. Web. Available here (www.khanacademy.com). 28 June 2017.
- Martha Sola-Visner. Hemostatic Challenges in Neonates.Patricia Davenport. Front.Pediatr., 02 March 2021,Sec. Pediatric Critical Care ,Volume 9 – 2021.
- Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood.,1992;80:1998–2005.
- Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, et al. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost,2006;95:362–72.
- Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, et al. Development of the humancoagulation system in the full-term infant .Blood,1987;70:165–72.
- M.Achey, U.Nag, V.Robinson, et al. The Developing Balance of Thrombosis and Hemorrhage in Pediatric Surgery Clinical Implications of Age-Related Changes in Hemostasis. Clinical and Applied Thrombosis/hemostasis,2020;26:1-12.
- Baer RD, Lambert DK, Henry E, Ilstrup SJ, Bennett ST.Reference intervals for common coagulation tests of preterm infants (CME).Transfusion,2014;54:627–32.