Kjaev I1, Anastasova S2, Milkovski D1, Nivichka J3, Karadjova D1, Aleskioska I1
1University Clinic for Gynecology and Obstetrics, Skopje
2University Clinic for Cardiology, Skopje
3University Clinic for Ophthalmology, Skopje
UDK: 618.3-06:616.12-008.331.1
https://www.doi.org/10.55302/MJA2481011k
Abstract
Introduction: Hypertensive disorders in pregnancy including preeclampsia are present in 10% of pregnancies and are one of the biggest reasons for both maternal and fetal morbidity and mortality.
Materials and Methods: The study was undertaken at the University Clinic for Gynecology and Obstetrics in Skopje, North Macedonia. After initial assessment, 81 patients were enrolled in the study after signing a written consent. Patients were divided into two groups depending on whether they had hypertension or not. In the hypertensive group 51 patients were enrolled and 30 normotensive pregnancies were used as controls.
Results: Based on the values of the parameters of diastolic function obtained with PDA of the transmittance flow and the values of the parameters obtained with TDI of the longitudinal movement of the mitral ring, diastolic dysfunction was found in 17 (33.2%) pregnant women of the studied population, LV function (p <0.001). In the pregnant women from the examined group in whom the presence of LV diastolic dysfunction was identified, the disorders were of mild degree, that is type of delayed relaxation of LV in all 17 pregnant women.
Conclusion: Early recognition and management of symptoms are essential. Women who suffer from hypertensive disorders in pregnancy require close monitoring after delivery.
Key Words: gestational hypertension, diastolic dysfunction, preeclampsia, pregnancy.
Introduction
Hypertensive disorders in pregnancy including preeclampsia are present in 10% of pregnancies and are one of the biggest reasons for both maternal and fetal morbidity/ mortality (1). According to the WHO (World Health Organization), 16% of maternal mortality in developing countries is due to pregnancy related hypertension. The key issue is that half of these could have been prevented if treated on time (2).
Pregnancy is a dynamic process associated with significant physiological changes in the cardiovascular system. Maternal inability to adapt to these physiological changes can expose underlying, previously silent, cardiac pathology, which is why some call pregnancy “the nature’s stress test”. Cardiovascular disease in pregnancy is the leading cause of maternal mortality in North America (3).
The hemodynamic changes in pregnancies complicated with hypertension depend on the type and severity of hypertension and previous chronic diseases. In some pregnancies cardiovascular changes can precede the actual hypertension (4).
The accurate assessment of cardiac function during pregnancy is important. In the past, studies on the maternal cardiovascular system focused mainly on systolic function (5). However, myocardial relaxation is an energy-dependent process and diastolic dysfunction has been shown to precede impairment of systolic function in the evolution of most of the cardiac diseases (6). New studies have suggested that diastolic dysfunction is a major cause of congestive heart failure, and the majority of people with congestive heart failure have preserved left ventricular systolic function (7).
The best method in evaluation of left ventricular (LV) diastolic function involves measurement of transmittal inflow velocity by pulsed wave Doppler echocardiography. It has been in use for the past several decades to evaluate heart function in pregnant women (8).
If we can identify any form of cardiac dysfunction along with its severity during early pregnancy, it may be possible to prevent progression of the condition and save mother from severe morbidity of acute heart failure (9). The aim of the study is to evaluate diastolic changes in pregnancies with gestational hypertension/ preeclampsia.
Materials and methods
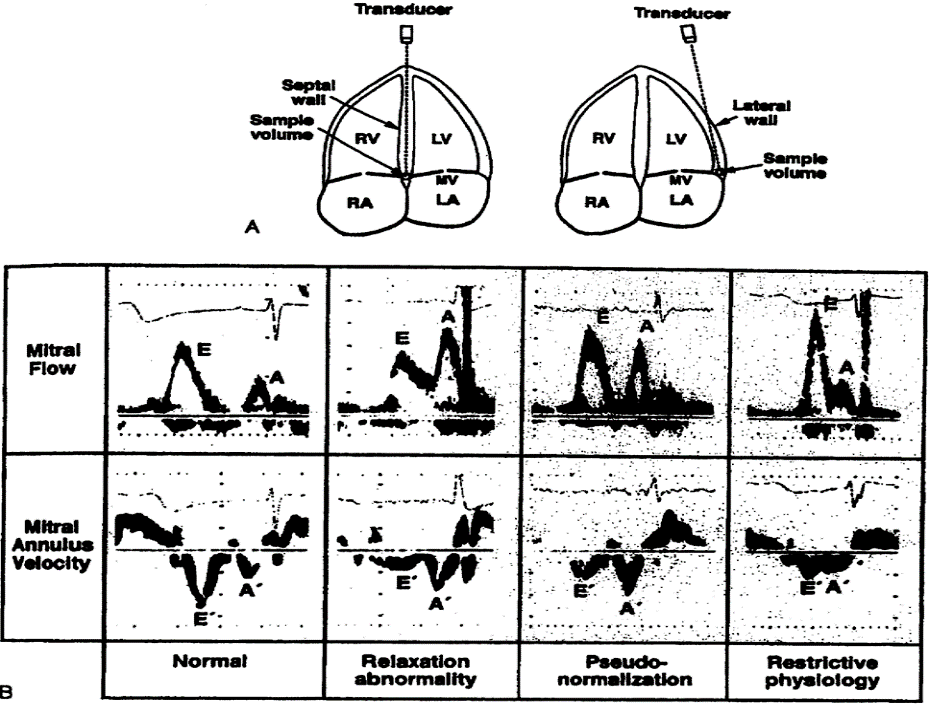
The study was undertaken at the University Clinic for Gynecology and Obstetrics in Skopje, Republic of North Macedonia. After initial assessment, 81 patients were enrolled in the study after signing a written consent. The patients were divided into two groups depending on whether they had hypertension or not. In the hypertensive group 51 patients were enrolled, and 30 normotensive pregnancies were used as controls. The initial examination was done at the 28th g.w. Subjects were classified as hypertensive if BP was ≥140/90 on two different occasion 6 hours apart.[1] The predetermined exclusion criteria for the study were: diabetes, maternal cardiovascular disease, alcohol and tobacco use. Gestation was confirmed by the last menstrual period and ultrasound measurement. BP was measured using the standard auscultatory method with the help of pneumatically operated mercurial type sphygmomanometer. BP was measured in the left arm in the sitting position with the arm at the level of the heart. Echocardiographic examination was done at the University Clinic for Cardiology with GE Vivid echocardiograph machine. M-mode studies were performed at the level of the aorta, left atrium and LV at mid position between the tips of the mitral valve. Systolic parameters studied were left ventricle end systolic diameter (LV ESD), left ventricle end diastolic diameter (LV EDD), stroke volume (SV), CO (cardiac output), left ventricular mass (LVM) and posterior wall thickness (PWT) in long-axis parasternal view. Diastolic parameters studied were E wave, A wave, E/A ratio, isovolumetric relaxation time (IVRT).
A total of 3 echocardiographies were made in the 28th g.w., 2 weeks after delivery and 6 months after delivery. The idea of the study was to determine heart’s function during and after pregnancy, to evaluate diastolic function even after the pregnancy has ended to see whether heart changes still exist. In patients with diastolic dysfunction 6 months postpartum further checkups were organized with cardiologists.
Table of Diastolic Changes
- Normal diastolic function,
- Disturbed (abnormal) relaxation or light abnormal relaxation,
- Pseudonormal type of diastolic dysfunction,
- Restrictive type of diastolic dysfunction.
Results
Statistical analyses of data were done with SPSS 21.0. For testing of normal distribution of data Shapiro -Wilk´s test was used. Categorical variables were shown with absolute and relative numbers and for describing quantitative variables descriptive statistics were used. For comparison of analyzed variables between evaluated and control group, Student’s t-test was used as a test of significance. For comparison of (0.1 and 2 control) Paired sample test was used. For describing categorical variables x2 test was used. The probability value (P < 0.05) is described as significant.
Table 1. Parameters of diastolic dysfunction determined with PDA (pulse doppler) on transmittal flow and pulse wave TDI e’ velocity (sm/sek) of longitudinal movement of the mitral ring in evaluated and control group.
| Evaluated group
(n=51) |
Control group
(n=30) |
P value |
|
| Е wave, m/s | 0.67±0.04 | 0.78±0.02 | < 0.005‡ |
| А wave, m/s | 0.62±0.12 | 0.57±0.04 | 0.05‡ |
| DТ, ms | 215.1±16.8 | 191±4.8 | < 0.005‡ |
| E/A ratio | 1.08±0.23 | 1.5±0.07 | <0.01‡ |
| E’, cm/s | 8.3±1.9 | 9.4±0.8 | <0.05‡ |
| E/E’ ratio | 9.9±2.0 | 8.2±0.5 | < 0.05‡ |
| PS, mm | 34.6±3.7 | 28.9±1.6 | <0.001‡ |
‡Student’s t-test
In Table 1, Doppler echocardiographic parameters define diastolic function in pregnancies with hypertension and in control group.
Student t-test has shown significant statistical difference between the two groups when Е wave speed (p<0.005), Е/А value ((p<0.01), DT(p<0.005), speed of longitudinal movement of the mitral ring – E’(p<0.05) and E/E’ value (p<0.05).
The Chi-square-test has shown statistical difference (p<0.001) between the two groups, in ratio to diastolic dysfunction, defined by PDA (pulse doppler) parameters and TDI velocity (Table 1).
Table 2. Diastolic function in both group defined by parameters of pulse wave (PDA) doppler of transmittal flow and TDI velocity (sm/ sek) of longitudinal movement of the mitral ring in evaluated and control group.
| Evaluated group
(n=51) |
Control group
(n=30) |
P | |
| Diastolic dysfunction | |||
| No (n, %) | 29(56.9%) | 29 (96.7%) | <0.001† |
| Yes (n, %) | 22(43.1%) | 1(3.3%) | |
| Delayed relaxation | 19 (37.2%) | 1 (3.3%) | |
| Pseudonormal type | 3 (5.9%) | 0 | |
| Restrictive type | 0 | 0 |
†Chi-square test
The Chi-square test showed us a statistically significant difference between the two groups, in terms of the presence of diastolic dysfunction of LV, defined according to the parameters of PDA.
Based on the values of the parameters of diastolic function obtained with PDA of the transmittal flow and the values of the parameters obtained with TDI of the longitudinal movement of the mitral ring, diastolic dysfunction was found in 22 (43.1%) pregnant women of the study population, in addition in the normotensive group diastolic dysfunction was found in 1 (3.3%) patient (p <0.001).
In patients in whom the presence of LV diastolic dysfunction was identified, the disorders were mild, that is a type of delayed relaxation of LV in 19 (37.2%) pregnant women, and moderate in 3 (5.9%) pregnant women. In the control group only one patient (3.3%) had delayed relaxation or a mild degree of diastolic dysfunction.
The first control was performed in all respondents 2 weeks postpartum.
Table 3.Parameters of diastolic dysfunction determined with PDA on transmittal flow and TDI (sm/ sek) of longitudinal movement of the mitral ring in evaluated and control group on the first control.
| Evaluated group
(n=51) |
Control group
(n=30) |
P value |
|
| Е wave, m/s | 0.67±0.04 | 0.77±0.02 | < 0.05‡ |
| А wave, m/s | 0.60±0.12 | 0.57±0.06 | 0.06‡ |
| DТ, ms | 208.3±18.8 | 196.5±5.3 | < 0.005‡ |
| E/A ratio | 1.28±0.23 | 1.52±0.07 | <0.01‡ |
| E’, cm/s | 8.7±1.6 | 9.6±0.7 | <0.05‡ |
| E/E’ ratio | 9.0±2.2 | 7.8±0.6 | < 0.05‡ |
| PS, mm | 33.5±4.1 | 28.9±1.6 | <0.001‡ |
‡Student’s t-test
Table 3 presents Doppler echocardiographic parameters that define diastolic function in pregnant women with preeclampsia or gestational hypertension and the control group (the first control).
Student t-test showed us a statistically significant difference between the two groups in terms of E wave velocity, E/ A ratio, DT, speed of the longitudinal movement of the mitral flow – E’i and E / E ‘ratio.
The Chi-square test showed a statistically significant difference (p <0.001) between the two groups, in terms of the prevalence of LV diastolic dysfunction, defined according to the parameters of PDA and TDA (Table 3).
Table 4. Diastolic function in both group, defined by parameters of transmittal profile, gained with PDA (pulse wave) and TDI of longitudinal movement of the mitral ring in evaluated and control group on the first control.
| Evaluated group
(n=51) |
Control group
(n=30) |
P | |
| Diastolic dysfunction | |||
| No (n, %) | 34(66.7%) | 30 (100%) | <0.001† |
| Yes (n, %) | 17(33.3%) | 0 | |
| Delayed relaxation | 17 (33.2%) | 0 | |
| Pseudonormal type | 0 | 0 | |
| Restrictive type | 0 | 0 |
†Chi-square test
The Chi-square test showed us a statistically significant difference between the two groups, in terms of the presence of diastolic dysfunction of LC, defined according to the parameters of PDA.
Based on the values of the parameters of diastolic function obtained with PDA of the transmittal flow and the values of the parameters obtained with TDI of the longitudinal movement of the mitral ring, diastolic dysfunction was found in 17 (33.2%) pregnant women of the study population. LV function (p <0.001). In the pregnant women from the examined group in whom the presence of LV diastolic dysfunction was identified, the disorders were of mild degree, that is type of delayed relaxation of LV in all 17 pregnant women.
The examined echocardiographic findings presented in Table 5 and 6 refer to the second control. This control was performed in all respondents 6 months postpartum. Unfortunately, some of the respondents did not respond to the last echocardiographic control, namely 8 patients from the study and 4 patients from the control group did not complete the second control. The same parameters were examined again.
Table 5. Parameters of diastolic dysfunction determined with PDA on transmittal flow and TDI of longitudinal movement of the mitral ring in evaluated and control group on second control.
| Evaluated group
(n=43) |
Control group
(n=26) |
P value |
|
| Е wave, m/s | 0.72±0.04 | 0.78±0.02 | < 0.05‡ |
| А wave, m/s | 0.59±0.12 | 0.56±0.14 | 0.06‡ |
| DТ, ms | 210.3±18.8 | 194.5±4.7 | < 0.05‡ |
| E/A ratio | 1.32±0.23 | 1.57±0.05 | <0.05‡ |
| E’, cm/s | 8.8±1.7 | 9.5±0.9 | 0.05‡ |
| E/E’ ratio | 8.6±2.5 | 7.6±0.6 | < 0.05‡ |
| PS, mm | 30.75±3.12 | 29.67±1.71 | 0.05‡ |
‡Student’s t-test
Table 6. Parameters of diastolic dysfunction determined with PDA (pulse doppler) on transmittal flow and TDI of longitudinal movement of the mitral ring in evaluated and control group on second control.
| Evaluated group
(n=43) |
Control group
(n=26) |
P | |
| Diastolic dysfunction | |||
| No (n, %) | 31(71.9%) | 26 (100%) | 0.003† |
| Yes (n, %) | 12 (28.1%) | 0 | |
| Abnormal relaxation | 17 (33.2%) | 0 | |
| Pseudonormal type | 0 | 0 | |
| Restrictive type | 0 | 0 |
†Chi-square test
We found diastolic dysfunction in 12 of the 43 subjects from the population with gestational hypertension or preeclampsia (28.1%), 8 pregnant women did not complete the study at the last control. In the control group 4 patients did not report at the last control and no positive finding was observed from the available respondents in this group (p <0.001) (Table 5).
Disscusion
The left ventricular diastolic dysfunction is defined as the inability of the heart to fill with normal blood volume without increasing ventricular filling pressure. Left ventricular diastolic function and left ventricular filling pressure were calculated and graded using standard diagnostic algorithms (11,12)
Diastolic dysfunction usually occurs before systolic dysfunction in the evolution of ischemic/ hypertensive cardiovascular disease and it is of prognostic importance in predicting long-term cardiovascular morbidity (13). The American College of Cardiology has highlighted the importance of identifying asymptomatic cardiac dysfunction for early intervention and improvement of outcome (14). Heart failure is a progressive condition, which begins with risk factors for left ventricular dysfunction and progresses further to asymptomatic changes in cardiac structure and function, finally evolving into heart failure (15). Diastolic dysfunction precedes the onset of systolic dysfunction in 50% of cardiac diseases, which further precedes the onset of heart failure (16).
The Olmsted study described the predictive significance of left ventricular diastolic dysfunction using multivariable adjusted analyses (17). One year post-delivery, diastolic dysfunction was present in 11.5% of women with pre-eclampsia, in 22.7% of women with early-onset pre-eclampsia and in 1.9% of women whose pre-eclampsia developed after 34 weeks.
In our study, diastolic dysfunction at the entrance of the study was identified in 43.1% of the evaluated pregnant women, at the first control the percentage decreased to 33.3% and at the last control – 6 months after delivery was 18.1%. In our study, pregnant women were not divided into early and late preeclampsia due to the low number of patients. In other studies, in preterm preeclampsia, diastolic dysfunction is found in more than 50% of cases at the entrance to the study (18). The one-year control study showed diastolic dysfunction in 14% of term preeclampsia compared to 40% in preterm preeclampsia. In our study, upon entering the study, diastolic dysfunction was identified in one pregnant woman in the control group, but it was not observed in the next two controls. In studies where diastolic dysfunction was observed in part of normotensive pregnant women, it normalized within 3 months after delivery (19). The changes that persist for more than a year have number of cardiovascular consequences, and these results seem to be specific to early onset preeclampsia (<34 g.w.), but not in term preeclampsia (20).
Conclusion
Early recognition and management of symptoms are essential. Women who suffer from hypertensive disorders in pregnancy require close monitoring after delivery. This has been shown especially in early onset preeclampsia. Up to 40% of those patients fit the criteria of B-stages heart failure (left ventricular diastolic dysfunction/ abnormal relaxation). These are young active women who don’t know that they have an underlying risk for chronic hypertension and future heart failure. Close cooperation between obstetricians and cardiologists is needed, so that these patients are not lost in the system.
Refferences:
- Bateman BT, Shaw KM, Kuklina EV, et al. Hypertension in women of reproductive age in the United States: NHANES 1999–2008. PLoS ONE 2012; 7(4): e36171.
- Wakis Ab,Saftlas AF,Hsia J,Atrash HK. Secular trends in the rates of preeclampsia,eclampsia and gestational hypertension,united states,1987-2004, Am J Hypertens 2008;21:521-6
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005.Obstet Gynecol. 2010; 116:1302–1309.
- Khalil A,Akolekar R,Syngelaki A, et al: Maternal hemodynamics at 11-13 weeks of gestations and risk of pre-eclampsia: Ultrasound Obstet Gynecol 40(1):28,2012
- Atkins AFJ, Watt JM, Milan P, Davies P, Crawford JS. A longitudinal study of cardiovascular dynamic changes throughout pregnancy. Eur J Obstet Gynecol Reprod Biol 1981; 12: 215–224.
- Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation 2002; 105: 1503–1508
- Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Cardiovascular Health Study Research Group. Importance of heart failure with preserved systolic function in patients ≥ 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 2001; 87: 413–419.
- Moran AM, Colan SD, Mauer MB, Geva T. Adaptive mechanisms of left ventricular diastolic function to the physiologic load of pregnancy. Clin Cardiol 2002; 25: 124–131
- Valensise H, Vasapollo B, Novelli GP, Pasqualetti P, Galante A, Arduini D. Maternal total vascular resistance and concentric geometry: a key to identify uncomplicated gestational hypertension. BJOG. 2006;113:1044–52
- Easterling TR, BenedeĴ i TJ, Schmucker BC, Millard SP. Maternal hemodynamic in normal and preeclamptic pregnancies: A longitudinal study. Obstet Gynecol 1990;76:1061-9
- Nagueh SF, Appelton CP, Gillbert TC and all.Recommendation for the evaluation of the left ventricular diastolic function by echocardiography. Eur J Echocardiography 2008;9:501-8
- Melchiorre K,Sutherland GR, Baltabaeva A and all. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension 2011;57:85-93
- Perk J, De Backer G , Gohlke H et all.Europeran Association for Cardiovascular prevention and Rehabilitation(EACRP);ESC committee for practice guidliness (CPG).European guideliness on cardiovascular disease prevention in clinical practice (version 2012).The fifith joint Task force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practise (constituted by representatives of nine societies and by invited experts) Eur Heart J 2012;33:1635-1701
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiatas TG. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. A report of the American College of Cardiology/American Heart Association Task Force on practical guidelines, 2005. Available at American College of Cardiology web-site (www.acc.org/qualityandscience/clinical/topic/ topic.htm).
- Kuznetsova T, Herbots L, Lopez B, Jin Y, Richart T, Thijs L. et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112.
- Melchiorre K, Thilaganathan B. Maternal cardiac function in preeclampsia. Curr Opin Obstet Gynecol. 2011;23:440–447.
- Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community. Appreciating the scope of the heart failure epidemic. J Am Med Assoc. 2003;289:194–202
- Melchiorre K, Sutherland G,Wart-Coote I et al:Severe myocardial imparement and chamber dysfunction in preterm preeclampsia.Hypertens pregnancy 31(4):454,2012
- Simmons L ,Gillin A ,Jeremy R.Structural and functional changes in left ventricle during normotensive and preeclamptic pregnancy. Am J Physiol Heart Circ Physiol 283 H1627-H1633 2002
- Bellamy I ,Casas JP, Hingorani AD et all. Pre-eclampsia and the risk of cardiovascular disease and cancer in latter life :systematic review and meta-analysis.BMJ 2007;335:974